Volume 11, Issue 4 (12-2023)
Jorjani Biomed J 2023, 11(4): 19-23 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Seifi F, Khajehlandi M. Effect of eight weeks of high-intensity interval training and moderate-intensity continuous training with quercetin supplementation on the gene expression of FOXO1 and ATG5 in the liver of diabetic obese rats. Jorjani Biomed J 2023; 11 (4) :19-23
URL: http://goums.ac.ir/jorjanijournal/article-1-1015-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-1015-en.html
1- Department of Exercise Physiology, Faculty of Educational Sciences and Psychology, University of Mohaghegh Ardabili, Ardabil, Iran , f.seifi@yahoo.com
2- Department of Exercise Physiology, Faculty of Educational Sciences and Psychology, University of Mohaghegh Ardabili, Ardabil, Iran
2- Department of Exercise Physiology, Faculty of Educational Sciences and Psychology, University of Mohaghegh Ardabili, Ardabil, Iran
Keywords: High-intensity interval training, Endurance Training, Autophagy, FOXO1 Protein, Human, ATG5 Protein, Human, Liver, Diabetes Mellitus, Type 2
Full-Text [PDF 612 kb]
(2411 Downloads)
| Abstract (HTML) (10086 Views)
Full-Text: (1375 Views)
Introduction
Today, increased consumption of a high-fat diet (HFD) and reduced physical activity are associated with metabolic complications such as obesity and metabolic syndrome. The process of increasing fat accumulation increases the infiltration of macrophages and, subsequently, chronic inflammation, which plays an essential role in insulin resistance (1,2). Thus, excessive fat accumulation is associated with a decrease in the sensitivity of glucose absorption and the re-esterification of free fatty acids (FFA), as well as an increase in lipolysis resistance due to the inhibitory effect of insulin in the abdominal and peripheral adipose tissue (3). Maintaining normal glucose homeostasis may be part of a plan to treat or prevent obesity and diabetes. Using animals for scientific purposes is a longstanding biological research and medicine practice. The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted researchers to investigate a large range of mechanisms and assess novel therapies in animal models before applying their discoveries to humans (4).
Numerous conflicting pathways make it difficult to activate and stop the synthesis of glucose because these pathways activate particular transcription factors, such as the forkhead box transcription factor O1 (FOXO1) (5). Substantial evidence indicates that FOXO1’s function depends on the modulation of downstream targets such as autophagy-associated genes, apoptosis, cell cycle arrest genes, anti-oxidative stress enzymes, and metabolic, as well as immune regulators (5,6). On the other hand, dysfunction of FOXO1 pathways leads to metabolic diseases such as diabetes, obesity, non-alcoholic fatty liver disease, and atherosclerosis (7). In obese or diabetic people, FOXO1-related gene expression increases some harmful characteristics related to obesity and diabetes, including hyperglycemia and glucose intolerance (8). In the liver, FOXO1 interacts with some other transcription factors such as PGC1α through its increasing role in the activity of enzymes or the expression of glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxylase (PEPC) genes to accelerate the process of gluconeogenesis, which leads to an increase in the release of hepatic glucose and an increase in blood glucose. Therefore, the acceleration of this process is accompanied by an increase in hepatic glucose release and hyperglycemia, especially in diabetic patients (9). Cells have a natural process called autophagy, which removes unnecessary or dysfunctional components through a lysosome-dependent regulated mechanism that is caused by various diseases, including diabetes (10). However, some existing research claims that the autophagy pathway may itself be impaired in diabetes (11). Autophagy-related 5 (ATG5) is one of the most commonly targeted genes in autophagy gene editing assays (12). Previous research found that obesity significantly reduced autophagy in the liver of both genetic and dietary mice models and that diabetes impacted hepatic autophagy. This effect was evidenced by lower expression levels of light chain 3 (LC3) and ATG5 (13). In line with previous studies, Xu et al. (2020) found that diabetes w:as char:acterized by lower expression of autophagy indicators in db/db mice livers, demonstrated by lower expression levels of LC3 and ATG5 proteins (14). Hence, the study of ATG5 is being considered in the context of diabetes.
Previous studies have shown that exercise training with different intensities and training protocols has different effects on fat oxidation (15-17). It has also been reported that the minimum intensity for affecting lipids is an intensity of 75% of the maximum heart rate (MHR). Performing exercise training and decreasing inflammation levels are associated with reduced risk of T2DM and obesity (18). Regular exercise training not only helps prevent T2DM but also improves diabetes-related indicators such as body mass index (BMI), blood glucose, insulin sensitivity, lipid profile, oxidative stress/antioxidant capacity, and chronic inflammation (19,20). Therefore, it is recommended to increase daily exercise and plan exercise routines as a part of the treatment for T2DM (20). It has been discovered that high-intensity interval training (HIIT) is superior to moderate-intensity continuous training (MICT) in terms of enhancing skeletal muscle strength, insulin sensitivity, mitochondrial biogenesis, glucose regulation, athletic ability, blood pressure, HDL-cholesterol, and cardiorespiratory fitness (21). Medicinal herbs are frequently used to treat T2DM. Reactive oxygen supplementation should be taken into consideration in T2DM patients in order to reduce increases in reactive oxygen species (ROS) after exercise. Flavonoids have demonstrated significant effects in the reduction of T2DM and prevention of cardiovascular disease (22). It is indicated that quercetin has beneficial effects, such as antioxidants, lowering blood glucose, dilating blood vessels, anti-inflammatory, anti-apoptotic, anti-atherogenic, and reducing blood lipids (23). Abnormal accumulation of lipid droplets is a vital hallmark of related diseases, including diabetes, obesity, and atherosclerosis. Therefore, for the diagnosis of diabetes, it is essential to develop a method of monitoring lipid droplets and viscosity simultaneously (24). The effect of different intensities and models of exercise with equal calorie consumption during exercise on the lipid droplets of the liver has not been fully investigated. Therefore, the aim of the present study was to investigate the effect of eight weeks of MICT and HIIT training with quercetin supplementation on the gene expression of FOXO1 and ATG5 in the liver of diabetic obese male Wistar rats.
Methods
Forty-two Wistar male rats (weight: 125-130 g, age: 8 weeks) were purchased from the Animal Care Center of the University of Mohaghegh Ardabil. The rats were divided into seven groups and six rats were placed in each group (n=6): healthy control group (HC), diabetes control group (DC), diabetic quercetin group (DCQ), high-intensity interval training with diabetes (DH), moderate-intensity continuous training with diabetes group (DM), diabetic high-intensity interval training with quercetin group (DHQ), and diabetic moderate-intensity continuous training with quercetin group (DMQ). Rats were housed in controlled environments with a light-dark cycle of 12 hours and an average temperature of 22±3 degrees Celsius. Diabetic, control, and healthy groups were kept in separate cages (3 rats in each cage). Eight weeks of HFD exposure and a modest dosage of 25 mg/kg i.p. streptozotocin (STZ) were utilized to produce T2DM. The best STZ dosage for HFD rats was chosen using data from a previous investigation (25). A volume equal to citrate buffer was also administered to the HC group of non-diabetic rats. To prepare HFD, 1% cholesterol powder, and 1% special 100% corn oil were added to the standard food (24). Rats with fasting blood glucose levels between 200 mg/dL and 400 mg/dL were classified as diabetic samples. One week after STZ administration, rats were given a small lancet wound on the tail vein and a drop of blood was placed on a glucometer strip. The strip was then measured by a glucometer device (Infopia Easy Gluco blood glucose monitor, South Korea). Inclusion criteria should not be confused with animal characteristics but can be related to these (e.g., body weights must be within a certain range for a particular procedure or blood glucose levels must be between 200 mg/dL and 400 mg/dL).
MICT & HIIT training protocols
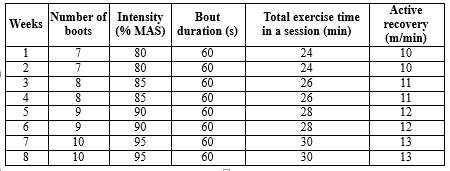
HIIT and MICT protocols, with a frequency of 5 times a week, totaling 40 training sessions, were performed on a treadmill. MICT exercise protocol consisted of a 10-minute warm-up to 33–49% of the rat’s maximal aerobic speed (MAS). Then, it was followed by 50 minutes of running at 65% MAS. The training ended with an active recovery of three minutes at 20–30% of the rat’s MAS (26). The highest running duration and speed were recorded to determine the values of MAS. The MAS values of each rat were determined at the beginning and after two, four, six, and eight weeks. The protocol for the MAS test involved an exercise session in which the starting speed of 10 m/min was progressively increased every 60 seconds by 3.33 m/min until reaching 26.7 m/min. The speed was then increased by 1.7 m/min until the rats could no longer continue running. (27). HIIT sessions consisted of 7–10 exercise bouts with an intensity between 80- 95% of MAS, which was followed by 60 seconds of active recovery with an intensity of 45–50% of MAS. Before and after each HIIT session, rats underwent the treadmill exercise for 5 min at 10 m/min warm-up and cool-down (Table 1).
Measurement of blood glucose and body weight
Before HFD and 48 hours following the most recent training session, the tail vein blood was obtained while on an overnight fast. Then, blood glucose concentration was directly assessed using a glucometer (Infopia Easy Gluco blood glucose monitor, South Korea). Values for body weight were taken before HFD exposure, following HFD, and 48 hours following the most recent training session. Blood samples were taken from the retro-orbital venous sinus using a standard test kit and centrifuged for five minutes at 5000 rpm to separate the serum.
The process of Real-time PCR
Rats were anesthetized by intraperitoneal injection of 20-30 mg/kg of 10% ketamine and 2-3 mg/kg of xylazine 2%. Then, the liver tissues were quickly separated, washed in a normal saline solution, and frozen in liquid nitrogen-free RNAase and DNAase microtubes to prevent any contamination for mRNA purification and real-time PCR. RNA was extracted using the Total RNA Extraction kit reagent in accordance with the manufacturer's guidelines (Pars Toss, Iran). After extracting RNA, real-time PCR was used to measure the expression of mRNA by the Lava 96 Real-time PCR Detection System (Daan Gene Co Ltd), and the kit used in the research was also 2X SYBR Green Real-Time PCR (Pars Toss, Iran). The real-time PCR reaction was performed with 6.25 microliters of master mix, 0.25 microliters of forward primer, 0.25 microliters of reverse primer, and 3 microliters of cDNA with 2.75 microliters of water. Comparative expression values of FOXO1 and ATG5 genes compared to the expression of GAPDH in each tissue were evaluated by Light Cycler SW1.1 software. The relationship 2-ΔΔCt was used for evaluation and reporting. Following the previously stated protocol, two repeats of the real-time PCR reaction were carried out on each sample, and each gene and two repetitions of the calculation of the average Ct values of various dilutions were made. Table 2 presents the sequence of the primers used in the present study to investigate FOXO1 and ATG5 gene expression.
Statistical analysis
Shapiro-Wilk and Levene’s tests confirmed the normality and the homogeneity of all the variances. The two-way analysis of variance test was used to investigate the difference in glucose changes and animal body weight, and the one-way analysis of variance (ANOVA) was used to test the values of gene expression change of FOXO1 and ATG5 between different groups after eight weeks of interventions. Tukey's post-hoc test was used to analyze the variance for the pair comparison. The statistical analyses were conducted using SPSS software version 26, with a significance level 0.05.
Results
Blood glucose and body weight after interventions
The results of the two-way analysis of variance test for blood glucose levels showed that the time effect (P-Value=0.001 and f=1597.48), group effect (P-Value=0.001 and f=67.37), and the interaction between time and group (P-Value=0.001 and f=52.92) was significant. The results of Tukey's post-hoc test showed that the induction of diabetes significantly increased the blood glucose levels of rats, but eight weeks of quercetin injection alone and combined with HIIT and MICT training and HIIT and MICT training alone controlled the blood levels of the diabetic rats (P-Value>0.001) (Figure 1). The results of the two-way analysis of variance test for the amount of weight changes showed that the time effect (P-Value=0.001 and f=6565.83), group effect (P-Value=0.001 and f=81.22) and the interaction of time and group (P-Value=0.001 and f=67.37) was significant. The results of Tukey's post-hoc test showed that the induction of obesity significantly increased the body weight levels of rats, but eight weeks of exercise intervention and quercetin injection, weight changes in DH, DM, DHQ, and DMQ groups were significantly decreased compared to DC and DCQ groups (P-Value<0.05) (Figure 2).
Gene expression of FOXO1 and ATG5 after interventions
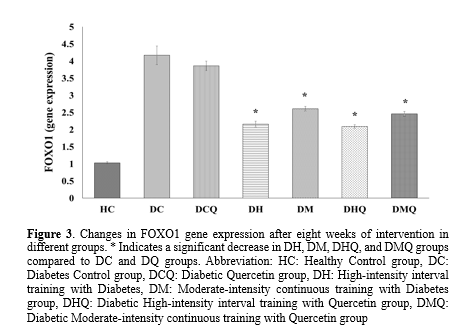
Since the sample we needed was liver tissue, we had one measurement for gene expression. In addition, training groups were compared with the control group to measure the changes after the investigation. The result of one-way ANOVA showed that there was a significant difference between seven groups for FOXO1 and ATG5 gene expression with the significance level of (P-Value=0.001) and (P-Value=0.001), respectively (Table 3). Induction of T2DM increased (3.14 unit) and decreased (0.7 unit) gene expression of FOXO1 (P-Value=0.001) and ATG5 (P-Value=0.001), respectively in liver tissue which was obtained by comparing the DC group with the HC group. The results of According to Tukey's post-hoc test for FOXO1 gene expression, there was a significant decrease in the DH group by 2.69 units, in the DM group by 2.01 units, in the DHQ group by 2.08 units, and in the DMQ group by 1.71 units, compared to the DC group (with a P-value of 0.001 for all groups). In addition, there was not any significant difference between all training groups with DHQ and DMQ and without quercetin DH and DM supplementation (P-Value>0.05) (Table 3 and Figure 3). According to the results of Tukey's post-hoc test for ATG5 gene expression, there was a significant increase in the DH (0.41 unit), DM (0.38 unit), DHQ (0.51 unit) and DMQ (0.42 unit) groups compared to the DC group. The p-values for these comparisons were all 0.001. There was no significant difference observed between all training groups with and without quercetin supplementation in DHQ and DMQ (P-Value>0.05) (Table 3 and Figure 4).
.PNG)
.PNG)

Discussion
This study aimed to investigate the impact of different-intensity exercise (HIIT and MICT) on the gene expression of FOXO1 and ATG5 in the liver of diabetic HFD-fed rats. The results showed that although inducing T2DM increased blood glucose levels, body weight, as well as gene expression of FOXO1, and decreased gene expression of ATF5 in the liver of diabetic obese rats, the implementation of MICT and HIIT exercises with and without quercetin supplementation for 8 weeks-controlled changes in the mentioned indicators. The most important finding of this study was the effect of quercetin on the blood glucose decreasing. In previous research, quercetin, one of the most widespread flavonoids in plants, has been reported as an effective factor in reducing the risk of T2DM in epidemiological research (28). Potential anti-diabetic effect of quercetin has been observed in in vivo and in vitro laboratory studies, which involves several basic mechanisms, including stimulation of insulin secretion, anti-oxidative and anti-inflammatory protection of the pancreas (29). In line with the result of the present study, it has been observed in a study that the intervention with quercetin in rats under a high-fat, high-carbohydrate diet caused a significant decrease in blood glucose (30). In general, polyphenols have potential effects on reducing insulin resistance by increasing the transfer of GLUT4 to the cell membrane of muscle and fat tissue, along with the induction of AMPK and PI3K pathways (31).
The importance of FOXO1 in the liver cells of diabetic patients is so great that some laboratory science researchers have pointed to its effective role in high glucose levels or fasting hyperglycemia related to hepatic gluconeogenesis. Regarding FOXO1, the results of most studies have shown that exercise training causes significant increase (32,33), but some studies showed no significant change or its decrease after exercise (33,34). In the present study its gene expression decreased by different intensity exercises that is consistent with the results of some studies (35,36), and inconsistent with others (37,38). In this regard, the study by Slopack et al. (2014) showed that long-term resistance training leads to the reduction of FOXO1 protein levels from the tenth session onwards (35). The study findings of Soheili et al. (2018) showed that resistance training caused a significant decrease in fasting glucose, insulin resistance, and FOXO1 gene expression in the subcutaneous fat tissue of diabetic rats compared to the control group, and it indicates that resistance training inhibition of FOXO1 gene expression in subcutaneous fat tissue leads to reduction of insulin resistance and serum glucose in diabetic rats (36). Contrary to the above-mentioned evidence, Karimi et al. (2018) showed that intense intermittent exercise caused an increase in the relative expression of FOXO1 in the pancreatic tissue of diabetic rats compared to the control group (37). There are different reasons for the differences between research results such as age of the subjects, gender, type of subject, tissue measured, type of training protocol. In addition, it can be said that the changes of FOXO1 in liver tissue and pancreas are not the same in response to exercise, it may be due to the different mechanisms of FOXO1 action in these tissues. Among the mechanisms of both exercises for decreasing in the gene expression of FOXO1, changes in SRA can be mentioned. SRA is a long non-coding RNA that has attracted increasing attention due to its important role in lipid metabolism. Mechanistically, aerobic exercise may inhibit FOXO1 transcriptional activity by repressing SRA expression. The results of a study showed that SRA plays an important role in aerobic exercise to improve liver fat metabolism. So, maybe one of the controlling mechanisms for FOXO1 gene expression is SRA, because it is probably a key potential lncRNA to improve inflammatory response to hepatic steatosis through MAPK signaling pathway (39). Based on the available evidence on the effective role of protein or BIF levels on hepatic glucose release, the decrease in blood glucose in the exercise group may be attributed to the decrease in FOXO1 expression caused by HIIT and MICT exercise in liver cells (40,41).
The other gene measured in the present study was ATG5 and the results showed that inducing T2DM decreased its gene expression levels, but 8 weeks of MICT and HIIT exercises with and without quercetin supplementation increased its gene expression in the liver of rats. In the field of ATG5 changes following exercise training, there are limited studies, and research results are different, as we can see in the study by Daneshyar et al. (2020) study results showed that six weeks of exercise did not change ATG5 levels (42) which is inconsistent with the current study result. While Pinto et al. (2021) investigated the comparison of a session of strength training, endurance training, training until exhaustion and concurrent training on TAG5 levels in the liver and heart of rats. The results showed that only exercise until exhaustion caused a significant decrease in this gene (43). There are different reasons for these different results such as the training protocol, the measured tissue, and the type of subjects exercising. The existence of genes related to autophagy is very necessary in the formation of auto phagosome in the signaling pathway. It has been suggested, one of the important factors for increasing the amount of cellular autophagy is exercise training: because exercise training causes stimulation such as hypoxia, possible structural damage to cell and increase or change of immune system factors increase autophagy (44). In relation to the increase of this gene following exercises in the present study, it is suspected that ATG5 is accompanied by high-fat nutrition and the accumulation and breakdown of triglycerides in fat cells of liver occur successively in the exercise condition. In this condition, the interaction of lipogenesis and the interaction of lipolysis and lipophagy are more involved, which is probably associated with an increase in the amount of autophagy (45). Because in this case, the probability of consumption of intracellular organelles and the accumulation of biological macromolecules (which are autophagy triggers) increases. Studies have shown that the mechanisms of lipid catabolism, i.e., lipolysis and lipophagy, are completely related to each other, and the regulatory connection point between these mechanisms is AMPK, which has been proven to be stimulated by exercise (39). Based on the findings of studies, it can be suggested that exercises which were prescribed in this study can probably induce the expression of the ATG5 gene in the liver tissue (46). In general, it can be assumed that the increasing effect caused by the combination of HFD and exercise training in the expression of ATG5, which is an important factor in autophagy, is probably related to the complex metabolic interactions related to the sequential anabolism and catabolism of lipids in adipose tissue. However, this theory needs more research in the future. Limitations of the present study are the lack of measuring protein levels of the two mentioned genes and other effective factors in the process of autophagy.
Conclusion
It was observed that both types of MICT and HIIT exercises with and without quercetin supplementation had significantly affected the blood glucose, body weight as well as FOXO1 and ATG5 gene expression. Quercetin supplementation although decreased blood glucose levels, it had not any significant effect on gene expression of FOXO1 and ATG5. So, considering the effect of quercetin on blood glucose control and the effect of both exercise training on improving gene expression, these interventions can be considered for controlling of diabetes.
Acknowledgement
The authors sincerely thank all persons who helped in the implementation of this research.
Funding sources
This study was taken from research project with the contract number of 1402//9/25538D and financial support approved by University of Mohaghegh Ardabili.
Ethical statement
The Ethics Committee of the University of Mohaghegh Ardabili approved this study’s protocol (IR.UMA.REC.1403.004).
Conflicts of interest
The equipment necessary for conducting the study was provided by University of Mohaghegh Ardabili. All authors were involved in data interpretation and presentation, and approved the final manuscript.
Author contributions
Mojdeh Khajehlandi played pivotal roles in data collection, statistical population collection and laboratory coordination. Farnaz Seifi and Mojdeh Khajehlandi pivotal roles in setting up the background of the research and their expertise and insights were crucial to the success of this research.
Today, increased consumption of a high-fat diet (HFD) and reduced physical activity are associated with metabolic complications such as obesity and metabolic syndrome. The process of increasing fat accumulation increases the infiltration of macrophages and, subsequently, chronic inflammation, which plays an essential role in insulin resistance (1,2). Thus, excessive fat accumulation is associated with a decrease in the sensitivity of glucose absorption and the re-esterification of free fatty acids (FFA), as well as an increase in lipolysis resistance due to the inhibitory effect of insulin in the abdominal and peripheral adipose tissue (3). Maintaining normal glucose homeostasis may be part of a plan to treat or prevent obesity and diabetes. Using animals for scientific purposes is a longstanding biological research and medicine practice. The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted researchers to investigate a large range of mechanisms and assess novel therapies in animal models before applying their discoveries to humans (4).
Numerous conflicting pathways make it difficult to activate and stop the synthesis of glucose because these pathways activate particular transcription factors, such as the forkhead box transcription factor O1 (FOXO1) (5). Substantial evidence indicates that FOXO1’s function depends on the modulation of downstream targets such as autophagy-associated genes, apoptosis, cell cycle arrest genes, anti-oxidative stress enzymes, and metabolic, as well as immune regulators (5,6). On the other hand, dysfunction of FOXO1 pathways leads to metabolic diseases such as diabetes, obesity, non-alcoholic fatty liver disease, and atherosclerosis (7). In obese or diabetic people, FOXO1-related gene expression increases some harmful characteristics related to obesity and diabetes, including hyperglycemia and glucose intolerance (8). In the liver, FOXO1 interacts with some other transcription factors such as PGC1α through its increasing role in the activity of enzymes or the expression of glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxylase (PEPC) genes to accelerate the process of gluconeogenesis, which leads to an increase in the release of hepatic glucose and an increase in blood glucose. Therefore, the acceleration of this process is accompanied by an increase in hepatic glucose release and hyperglycemia, especially in diabetic patients (9). Cells have a natural process called autophagy, which removes unnecessary or dysfunctional components through a lysosome-dependent regulated mechanism that is caused by various diseases, including diabetes (10). However, some existing research claims that the autophagy pathway may itself be impaired in diabetes (11). Autophagy-related 5 (ATG5) is one of the most commonly targeted genes in autophagy gene editing assays (12). Previous research found that obesity significantly reduced autophagy in the liver of both genetic and dietary mice models and that diabetes impacted hepatic autophagy. This effect was evidenced by lower expression levels of light chain 3 (LC3) and ATG5 (13). In line with previous studies, Xu et al. (2020) found that diabetes w:as char:acterized by lower expression of autophagy indicators in db/db mice livers, demonstrated by lower expression levels of LC3 and ATG5 proteins (14). Hence, the study of ATG5 is being considered in the context of diabetes.
Previous studies have shown that exercise training with different intensities and training protocols has different effects on fat oxidation (15-17). It has also been reported that the minimum intensity for affecting lipids is an intensity of 75% of the maximum heart rate (MHR). Performing exercise training and decreasing inflammation levels are associated with reduced risk of T2DM and obesity (18). Regular exercise training not only helps prevent T2DM but also improves diabetes-related indicators such as body mass index (BMI), blood glucose, insulin sensitivity, lipid profile, oxidative stress/antioxidant capacity, and chronic inflammation (19,20). Therefore, it is recommended to increase daily exercise and plan exercise routines as a part of the treatment for T2DM (20). It has been discovered that high-intensity interval training (HIIT) is superior to moderate-intensity continuous training (MICT) in terms of enhancing skeletal muscle strength, insulin sensitivity, mitochondrial biogenesis, glucose regulation, athletic ability, blood pressure, HDL-cholesterol, and cardiorespiratory fitness (21). Medicinal herbs are frequently used to treat T2DM. Reactive oxygen supplementation should be taken into consideration in T2DM patients in order to reduce increases in reactive oxygen species (ROS) after exercise. Flavonoids have demonstrated significant effects in the reduction of T2DM and prevention of cardiovascular disease (22). It is indicated that quercetin has beneficial effects, such as antioxidants, lowering blood glucose, dilating blood vessels, anti-inflammatory, anti-apoptotic, anti-atherogenic, and reducing blood lipids (23). Abnormal accumulation of lipid droplets is a vital hallmark of related diseases, including diabetes, obesity, and atherosclerosis. Therefore, for the diagnosis of diabetes, it is essential to develop a method of monitoring lipid droplets and viscosity simultaneously (24). The effect of different intensities and models of exercise with equal calorie consumption during exercise on the lipid droplets of the liver has not been fully investigated. Therefore, the aim of the present study was to investigate the effect of eight weeks of MICT and HIIT training with quercetin supplementation on the gene expression of FOXO1 and ATG5 in the liver of diabetic obese male Wistar rats.
Methods
Forty-two Wistar male rats (weight: 125-130 g, age: 8 weeks) were purchased from the Animal Care Center of the University of Mohaghegh Ardabil. The rats were divided into seven groups and six rats were placed in each group (n=6): healthy control group (HC), diabetes control group (DC), diabetic quercetin group (DCQ), high-intensity interval training with diabetes (DH), moderate-intensity continuous training with diabetes group (DM), diabetic high-intensity interval training with quercetin group (DHQ), and diabetic moderate-intensity continuous training with quercetin group (DMQ). Rats were housed in controlled environments with a light-dark cycle of 12 hours and an average temperature of 22±3 degrees Celsius. Diabetic, control, and healthy groups were kept in separate cages (3 rats in each cage). Eight weeks of HFD exposure and a modest dosage of 25 mg/kg i.p. streptozotocin (STZ) were utilized to produce T2DM. The best STZ dosage for HFD rats was chosen using data from a previous investigation (25). A volume equal to citrate buffer was also administered to the HC group of non-diabetic rats. To prepare HFD, 1% cholesterol powder, and 1% special 100% corn oil were added to the standard food (24). Rats with fasting blood glucose levels between 200 mg/dL and 400 mg/dL were classified as diabetic samples. One week after STZ administration, rats were given a small lancet wound on the tail vein and a drop of blood was placed on a glucometer strip. The strip was then measured by a glucometer device (Infopia Easy Gluco blood glucose monitor, South Korea). Inclusion criteria should not be confused with animal characteristics but can be related to these (e.g., body weights must be within a certain range for a particular procedure or blood glucose levels must be between 200 mg/dL and 400 mg/dL).
MICT & HIIT training protocols
HIIT and MICT protocols, with a frequency of 5 times a week, totaling 40 training sessions, were performed on a treadmill. MICT exercise protocol consisted of a 10-minute warm-up to 33–49% of the rat’s maximal aerobic speed (MAS). Then, it was followed by 50 minutes of running at 65% MAS. The training ended with an active recovery of three minutes at 20–30% of the rat’s MAS (26). The highest running duration and speed were recorded to determine the values of MAS. The MAS values of each rat were determined at the beginning and after two, four, six, and eight weeks. The protocol for the MAS test involved an exercise session in which the starting speed of 10 m/min was progressively increased every 60 seconds by 3.33 m/min until reaching 26.7 m/min. The speed was then increased by 1.7 m/min until the rats could no longer continue running. (27). HIIT sessions consisted of 7–10 exercise bouts with an intensity between 80- 95% of MAS, which was followed by 60 seconds of active recovery with an intensity of 45–50% of MAS. Before and after each HIIT session, rats underwent the treadmill exercise for 5 min at 10 m/min warm-up and cool-down (Table 1).
|
Table 1. HIIT training protocol
 |
Before HFD and 48 hours following the most recent training session, the tail vein blood was obtained while on an overnight fast. Then, blood glucose concentration was directly assessed using a glucometer (Infopia Easy Gluco blood glucose monitor, South Korea). Values for body weight were taken before HFD exposure, following HFD, and 48 hours following the most recent training session. Blood samples were taken from the retro-orbital venous sinus using a standard test kit and centrifuged for five minutes at 5000 rpm to separate the serum.
The process of Real-time PCR
Rats were anesthetized by intraperitoneal injection of 20-30 mg/kg of 10% ketamine and 2-3 mg/kg of xylazine 2%. Then, the liver tissues were quickly separated, washed in a normal saline solution, and frozen in liquid nitrogen-free RNAase and DNAase microtubes to prevent any contamination for mRNA purification and real-time PCR. RNA was extracted using the Total RNA Extraction kit reagent in accordance with the manufacturer's guidelines (Pars Toss, Iran). After extracting RNA, real-time PCR was used to measure the expression of mRNA by the Lava 96 Real-time PCR Detection System (Daan Gene Co Ltd), and the kit used in the research was also 2X SYBR Green Real-Time PCR (Pars Toss, Iran). The real-time PCR reaction was performed with 6.25 microliters of master mix, 0.25 microliters of forward primer, 0.25 microliters of reverse primer, and 3 microliters of cDNA with 2.75 microliters of water. Comparative expression values of FOXO1 and ATG5 genes compared to the expression of GAPDH in each tissue were evaluated by Light Cycler SW1.1 software. The relationship 2-ΔΔCt was used for evaluation and reporting. Following the previously stated protocol, two repeats of the real-time PCR reaction were carried out on each sample, and each gene and two repetitions of the calculation of the average Ct values of various dilutions were made. Table 2 presents the sequence of the primers used in the present study to investigate FOXO1 and ATG5 gene expression.
|
Table 2. The sequence of primers for quantitative real-time PCR
.PNG) |
Shapiro-Wilk and Levene’s tests confirmed the normality and the homogeneity of all the variances. The two-way analysis of variance test was used to investigate the difference in glucose changes and animal body weight, and the one-way analysis of variance (ANOVA) was used to test the values of gene expression change of FOXO1 and ATG5 between different groups after eight weeks of interventions. Tukey's post-hoc test was used to analyze the variance for the pair comparison. The statistical analyses were conducted using SPSS software version 26, with a significance level 0.05.
Results
Blood glucose and body weight after interventions
The results of the two-way analysis of variance test for blood glucose levels showed that the time effect (P-Value=0.001 and f=1597.48), group effect (P-Value=0.001 and f=67.37), and the interaction between time and group (P-Value=0.001 and f=52.92) was significant. The results of Tukey's post-hoc test showed that the induction of diabetes significantly increased the blood glucose levels of rats, but eight weeks of quercetin injection alone and combined with HIIT and MICT training and HIIT and MICT training alone controlled the blood levels of the diabetic rats (P-Value>0.001) (Figure 1). The results of the two-way analysis of variance test for the amount of weight changes showed that the time effect (P-Value=0.001 and f=6565.83), group effect (P-Value=0.001 and f=81.22) and the interaction of time and group (P-Value=0.001 and f=67.37) was significant. The results of Tukey's post-hoc test showed that the induction of obesity significantly increased the body weight levels of rats, but eight weeks of exercise intervention and quercetin injection, weight changes in DH, DM, DHQ, and DMQ groups were significantly decreased compared to DC and DCQ groups (P-Value<0.05) (Figure 2).
Gene expression of FOXO1 and ATG5 after interventions
Since the sample we needed was liver tissue, we had one measurement for gene expression. In addition, training groups were compared with the control group to measure the changes after the investigation. The result of one-way ANOVA showed that there was a significant difference between seven groups for FOXO1 and ATG5 gene expression with the significance level of (P-Value=0.001) and (P-Value=0.001), respectively (Table 3). Induction of T2DM increased (3.14 unit) and decreased (0.7 unit) gene expression of FOXO1 (P-Value=0.001) and ATG5 (P-Value=0.001), respectively in liver tissue which was obtained by comparing the DC group with the HC group. The results of According to Tukey's post-hoc test for FOXO1 gene expression, there was a significant decrease in the DH group by 2.69 units, in the DM group by 2.01 units, in the DHQ group by 2.08 units, and in the DMQ group by 1.71 units, compared to the DC group (with a P-value of 0.001 for all groups). In addition, there was not any significant difference between all training groups with DHQ and DMQ and without quercetin DH and DM supplementation (P-Value>0.05) (Table 3 and Figure 3). According to the results of Tukey's post-hoc test for ATG5 gene expression, there was a significant increase in the DH (0.41 unit), DM (0.38 unit), DHQ (0.51 unit) and DMQ (0.42 unit) groups compared to the DC group. The p-values for these comparisons were all 0.001. There was no significant difference observed between all training groups with and without quercetin supplementation in DHQ and DMQ (P-Value>0.05) (Table 3 and Figure 4).
.PNG)
.PNG)
|
Table 3. The results of one-way analysis of variance and Tukey's post-hoc test for two-by-two comparison of groups for FOXO1 and ATG5 gene expression
.PNG) |


Discussion
This study aimed to investigate the impact of different-intensity exercise (HIIT and MICT) on the gene expression of FOXO1 and ATG5 in the liver of diabetic HFD-fed rats. The results showed that although inducing T2DM increased blood glucose levels, body weight, as well as gene expression of FOXO1, and decreased gene expression of ATF5 in the liver of diabetic obese rats, the implementation of MICT and HIIT exercises with and without quercetin supplementation for 8 weeks-controlled changes in the mentioned indicators. The most important finding of this study was the effect of quercetin on the blood glucose decreasing. In previous research, quercetin, one of the most widespread flavonoids in plants, has been reported as an effective factor in reducing the risk of T2DM in epidemiological research (28). Potential anti-diabetic effect of quercetin has been observed in in vivo and in vitro laboratory studies, which involves several basic mechanisms, including stimulation of insulin secretion, anti-oxidative and anti-inflammatory protection of the pancreas (29). In line with the result of the present study, it has been observed in a study that the intervention with quercetin in rats under a high-fat, high-carbohydrate diet caused a significant decrease in blood glucose (30). In general, polyphenols have potential effects on reducing insulin resistance by increasing the transfer of GLUT4 to the cell membrane of muscle and fat tissue, along with the induction of AMPK and PI3K pathways (31).
The importance of FOXO1 in the liver cells of diabetic patients is so great that some laboratory science researchers have pointed to its effective role in high glucose levels or fasting hyperglycemia related to hepatic gluconeogenesis. Regarding FOXO1, the results of most studies have shown that exercise training causes significant increase (32,33), but some studies showed no significant change or its decrease after exercise (33,34). In the present study its gene expression decreased by different intensity exercises that is consistent with the results of some studies (35,36), and inconsistent with others (37,38). In this regard, the study by Slopack et al. (2014) showed that long-term resistance training leads to the reduction of FOXO1 protein levels from the tenth session onwards (35). The study findings of Soheili et al. (2018) showed that resistance training caused a significant decrease in fasting glucose, insulin resistance, and FOXO1 gene expression in the subcutaneous fat tissue of diabetic rats compared to the control group, and it indicates that resistance training inhibition of FOXO1 gene expression in subcutaneous fat tissue leads to reduction of insulin resistance and serum glucose in diabetic rats (36). Contrary to the above-mentioned evidence, Karimi et al. (2018) showed that intense intermittent exercise caused an increase in the relative expression of FOXO1 in the pancreatic tissue of diabetic rats compared to the control group (37). There are different reasons for the differences between research results such as age of the subjects, gender, type of subject, tissue measured, type of training protocol. In addition, it can be said that the changes of FOXO1 in liver tissue and pancreas are not the same in response to exercise, it may be due to the different mechanisms of FOXO1 action in these tissues. Among the mechanisms of both exercises for decreasing in the gene expression of FOXO1, changes in SRA can be mentioned. SRA is a long non-coding RNA that has attracted increasing attention due to its important role in lipid metabolism. Mechanistically, aerobic exercise may inhibit FOXO1 transcriptional activity by repressing SRA expression. The results of a study showed that SRA plays an important role in aerobic exercise to improve liver fat metabolism. So, maybe one of the controlling mechanisms for FOXO1 gene expression is SRA, because it is probably a key potential lncRNA to improve inflammatory response to hepatic steatosis through MAPK signaling pathway (39). Based on the available evidence on the effective role of protein or BIF levels on hepatic glucose release, the decrease in blood glucose in the exercise group may be attributed to the decrease in FOXO1 expression caused by HIIT and MICT exercise in liver cells (40,41).
The other gene measured in the present study was ATG5 and the results showed that inducing T2DM decreased its gene expression levels, but 8 weeks of MICT and HIIT exercises with and without quercetin supplementation increased its gene expression in the liver of rats. In the field of ATG5 changes following exercise training, there are limited studies, and research results are different, as we can see in the study by Daneshyar et al. (2020) study results showed that six weeks of exercise did not change ATG5 levels (42) which is inconsistent with the current study result. While Pinto et al. (2021) investigated the comparison of a session of strength training, endurance training, training until exhaustion and concurrent training on TAG5 levels in the liver and heart of rats. The results showed that only exercise until exhaustion caused a significant decrease in this gene (43). There are different reasons for these different results such as the training protocol, the measured tissue, and the type of subjects exercising. The existence of genes related to autophagy is very necessary in the formation of auto phagosome in the signaling pathway. It has been suggested, one of the important factors for increasing the amount of cellular autophagy is exercise training: because exercise training causes stimulation such as hypoxia, possible structural damage to cell and increase or change of immune system factors increase autophagy (44). In relation to the increase of this gene following exercises in the present study, it is suspected that ATG5 is accompanied by high-fat nutrition and the accumulation and breakdown of triglycerides in fat cells of liver occur successively in the exercise condition. In this condition, the interaction of lipogenesis and the interaction of lipolysis and lipophagy are more involved, which is probably associated with an increase in the amount of autophagy (45). Because in this case, the probability of consumption of intracellular organelles and the accumulation of biological macromolecules (which are autophagy triggers) increases. Studies have shown that the mechanisms of lipid catabolism, i.e., lipolysis and lipophagy, are completely related to each other, and the regulatory connection point between these mechanisms is AMPK, which has been proven to be stimulated by exercise (39). Based on the findings of studies, it can be suggested that exercises which were prescribed in this study can probably induce the expression of the ATG5 gene in the liver tissue (46). In general, it can be assumed that the increasing effect caused by the combination of HFD and exercise training in the expression of ATG5, which is an important factor in autophagy, is probably related to the complex metabolic interactions related to the sequential anabolism and catabolism of lipids in adipose tissue. However, this theory needs more research in the future. Limitations of the present study are the lack of measuring protein levels of the two mentioned genes and other effective factors in the process of autophagy.
Conclusion
It was observed that both types of MICT and HIIT exercises with and without quercetin supplementation had significantly affected the blood glucose, body weight as well as FOXO1 and ATG5 gene expression. Quercetin supplementation although decreased blood glucose levels, it had not any significant effect on gene expression of FOXO1 and ATG5. So, considering the effect of quercetin on blood glucose control and the effect of both exercise training on improving gene expression, these interventions can be considered for controlling of diabetes.
Acknowledgement
The authors sincerely thank all persons who helped in the implementation of this research.
Funding sources
This study was taken from research project with the contract number of 1402//9/25538D and financial support approved by University of Mohaghegh Ardabili.
Ethical statement
The Ethics Committee of the University of Mohaghegh Ardabili approved this study’s protocol (IR.UMA.REC.1403.004).
Conflicts of interest
The equipment necessary for conducting the study was provided by University of Mohaghegh Ardabili. All authors were involved in data interpretation and presentation, and approved the final manuscript.
Author contributions
Mojdeh Khajehlandi played pivotal roles in data collection, statistical population collection and laboratory coordination. Farnaz Seifi and Mojdeh Khajehlandi pivotal roles in setting up the background of the research and their expertise and insights were crucial to the success of this research.
Type of Article: Original article |
Subject:
Health
Received: 2023/09/28 | Accepted: 2023/12/10 | Published: 2023/12/30
Received: 2023/09/28 | Accepted: 2023/12/10 | Published: 2023/12/30
References
1. Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci. 2022;23(2):786. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Litwin M, Kułaga Z. Obesity, metabolic syndrome, and primary hypertension. Pediatr Nephrol. 2021;36(4):825-37. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Gastaldelli A, Miyazaki Y, Pettiti M, Matsuda M, Mahankali S, Santini E, et al. Metabolic effects of visceral fat accumulation in type 2 diabetes. J Clin Endocrinol Metab. 2002;87(11):5098-103. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Barré-Sinoussi F, Montagutelli X. Animal models are essential to biological research: issues and perspectives. Future Sci OA. 2015;1(4):FSO63. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Benchoula K, Arya A, Parhar IS, Hwa WE. FoxO1 signaling as a therapeutic target for type 2 diabetes and obesity. European journal of pharmacology. 2021;891:173758. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Xing Y-q, Li A, Yang Y, Li X-x, Zhang L-n, Guo H-c. The regulation of FOXO1 and its role in disease progression. Life sciences. 2018;193:124-31. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Berlanga A, Guiu-Jurado E, Porras JA, Auguet T. Molecular pathways in non-alcoholic fatty liver disease. Clin Exp Gastroenterol. 2014;7:221-39. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Li Y, Ma Z, Jiang S, Hu W, Li T, Di S, et al. A global perspective on FOXO1 in lipid metabolism and lipid-related diseases. Prog Lipid Res. 2017;66:42-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Xiong X, Tao R, DePinho RA, Dong XC. Deletion of hepatic FoxO1/3/4 genes in mice significantly impacts on glucose metabolism through downregulation of gluconeogenesis and upregulation of glycolysis. PloS One. 2013;8(8):e74340. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Yang D, Yang Y, Li Y, Han R. Physical exercise as therapy for type 2 diabetes mellitus: From mechanism to orientation. Ann Nutr Metab. 2019;74(4):313-21. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Møller AB, Kampmann U, Hedegaard J, Thorsen K, Nordentoft I, Vendelbo MH, et al. Altered gene expression and repressed markers of autophagy in skeletal muscle of insulin resistant patients with type 2 diabetes. Sci Rep. 2017;7(1):43775. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Changotra H, Kaur S, Yadav SS, Gupta GL, Parkash J, Duseja A. ATG5: A central autophagy regulator implicated in various human diseases. Cell Biochem Funct. 2022;40(7):650-67. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11(6):467-78. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Xu Z, Wu Y, Wang F, Li X, Wang P, Li Y, et al. Fibroblast growth factor 1 ameliorates diabetes-induced liver injury by reducing cellular stress and restoring autophagy. Front Pharmacol. 2020;11:52. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Amati F, Dubé JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60(10):2588-97. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Shaw CS, Shepherd SO, Wagenmakers AJ, Hansen D, Dendale P, Van Loon LJ. Prolonged exercise training increases intramuscular lipid content and perilipin 2 expression in type I muscle fibers of patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2012;303(9):E1158-65. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Alvarez-Jimenez L, Morales-Palomo F, Moreno-Cabañas A, Ortega JF, Mora-Rodriguez R. Effects of statins on fat oxidation improvements after aerobic exercise training. J Clin Endocrinol Metab. 2023;108(5):e139-47. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Cereijo L, Gullón P, Del Cura I, Valadés D, Bilal U, Badland H, et al. Exercise facilities and the prevalence of obesity and type 2 diabetes in the city of Madrid. Diabetologia. 2022;65(1):150-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Bennetsen SL, Feineis CS, Legaard GE, Lyngbæk MP, Karstoft K, Ried-Larsen M. The impact of physical activity on glycemic variability assessed by continuous glucose monitoring in patients with type 2 diabetes mellitus: a systematic review. Front Endocrinol (Lausanne). 2020;11:486. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Esefeld K, Kress S, Behrens M, Zimmer P, Stumvoll M, Thurm U, et al. Diabetes, sports and exercise. Experimental and Clinical Endocrinology & Diabetes. 2021;129(S 01):S52-S9. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Yu H, Zhao X, Wu X, Yang J, Wang J, Hou L. High-intensity interval training versus moderate-intensity continuous training on patient quality of life in cardiovascular disease: A systematic review and meta-analysis. Sci Rep. 2023;13(1):13915. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Umeno A, Horie M, Murotomi K, Nakajima Y, Yoshida Y. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules. 2016;21(6):708. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Chiş I, Baltaru D, Dumitrovici A, Coseriu A, Radu B, Moldovan R, et al. Protective effects of quercetin from oxidative/nitrosative stress under intermittent hypobaric hypoxia exposure in the rat's heart. Physiol Int. 2018;105(3):233-46. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Wang H, Zheng H, Zhang W, Yang L, Yu M, Li Z. A near-infrared aggregation-induced emission probe for imaging lipid droplet and in vivo visualization of diabetes-related viscosity variations. Sens Actuators B Chem. 2023;394:134347. [View at Publisher] [DOI] [Google Scholar]
25. Veerapur V, Prabhakar K, Thippeswamy B, Bansal P, Srinivasan K, Unnikrishnan M. Antidiabetic effect of Ficus racemosa Linn. stem bark in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rats: a mechanistic study. Food Chem. 2012;132(1):186-93. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Pengam M, Goanvec C, Moisan C, Simon B, Albacète G, Féray A, et al. Moderate intensity continuous versus high intensity interval training: Metabolic responses of slow and fast skeletal muscles in rat. PloS One. 2023;18(10):e0292225. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Dupas J, Feray A, Guernec A, Pengam M, Inizan M, Guerrero F, et al. Effect of personalized moderate exercise training on Wistar rats fed with a fructose enriched water. Nutr Metab (Lond). 2018;15(1):69. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Yao Z, Gu Y, Zhang Q, Liu L, Meng G, Wu H, et al. Estimated daily quercetin intake and association with the prevalence of type 2 diabetes mellitus in Chinese adults. Eur J Nutr. 2019;58(2):819-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Michala A-S, Pritsa A. Quercetin: a molecule of great biochemical and clinical value and its beneficial effect on diabetes and cancer. Diseases. 2022;10(3):37. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Ansari P, Choudhury ST, Seidel V, Rahman AB, Aziz MA, Richi AE, et al. Therapeutic potential of quercetin in the management of type-2 diabetes mellitus. Life (Basel). 2022;12(8):1146. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Bahadoran Z, Golzarand M, Mirmiran P, Saadati N, Azizi F. The association of dietary phytochemical index and cardiometabolic risk factors in adults: Tehran Lipid and Glucose Study. J Hum Nutr Diet. 2013;26(s1):145-53. [view at publisher] [DOI] [PMID] [Google Scholar]
32. Kostić M, Korićanac G, Tepavčević S, Stanišić J, Romić S, Ćulafić T, et al. Low-intensity exercise affects cardiac fatty acid oxidation by increasing the nuclear content of pparα, foxo1, and lipin1 in fructose-fed rats. Metab Syndr Relat Disord. 2023;21(2):122-31. [view at publisher] [DOI] [PMID] [Google Scholar]
33. Pereira RM, da Cruz Rodrigues KC, Sant'Ana MR, da Rocha AL, Morelli AP, Veras AS, et al. FOXO1 is downregulated in obese mice subjected to short‐term strength training. J Cell Physiol. 2022;237(11):4262-74. [view at publisher] [DOI] [PMID] [Google Scholar]
34. Yarmohammadi M, Behboudi L, Eizadi M. The Effect of 12 Weeks Resistance Training on FOXO1 Expression in Hepatocytes, Glucose and Insulin in Diabetic Rats-A Brief-Report. Iranian journal of diabetes and obesity. 2020;11(3):193-5. [view at publisher] [DOI] [Google Scholar]
35. Slopack D, Roudier E, Liu ST, Nwadozi E, Birot O, Haas TL. Forkhead BoxO transcription factors restrain exercise‐induced angiogenesis. J Physiol. 2014;592(18):4069-82. [view at publisher] [DOI] [PMID] [Google Scholar]
36. Sohaily S, Eizadi M, Tarmast D. Effect of resistance training on FOXO1 gene expression in subcutaneous fatty tissue in diabetic wistar rats. J Gorgan Univ Med Sci. 2019;21(4):53-9. [view at publisher] [Google Scholar]
37. Karimi M, Eizadi M. The effect of interval training on FOXO1 expression in pancreas tissue of diabetes rats with high fat diet and STZ. Razi Journal of Medical Sciences. 2019;26(6):95-104. [view at publisher] [Google Scholar]
38. Joodi M, Banaeifar A, Eizadia M, Arshadi S. The changes of serum insulin in response to resistance training with emphasis on FOXO1 in pancreas of diabetes rats. J Jiroft Univ Med Sci. 2021;7(4):480-8. [view at publisher] [Google Scholar]
39. Wu B, Xu C, Tian Y, Zeng Y, Yan F, Chen A, et al. Aerobic exercise promotes the expression of ATGL and attenuates inflammation to improve hepatic steatosis via lncRNA SRA. Sci Rep. 2022;12(1):5370. [view at publisher] [DOI] [PMID] [Google Scholar]
40. Kamagate A, Kim DH, Zhang T, Slusher S, Gramignoli R, Strom SC, et al. FoxO1 links hepatic insulin action to endoplasmic reticulum stress. Endocrinology. 2010;151(8):3521-35. [view at publisher] [DOI] [PMID] [Google Scholar]
41. Kazeminasab F, Baharlooie M, Rezazadeh H, Soltani N, Rosenkranz SK. The effects of aerobic exercise on liver function, insulin resistance, and lipid profiles in prediabetic and type 2 diabetic mice. Physiol Behav. 2023;271:114340 [view at publisher] [DOI] [PMID] [Google Scholar]
42. Daneshyar S, Pouyandeh Ravan A, Khosravi A, Fourotan Y. The Long-Term Effect of High Fat Diet and Regular Aerobic Exercise Training on Gene Expression of Isoforms of Mitochondrial Creatine Kinase (Ckmt1, 2) in White Adipose Tissue of Mice: An Experimental Study. J Rafsanjan Univ Med Sci. 2020;19(6):619-32. [view at publisher] [Google Scholar]
43. Pinto AP, da Rocha AL, Marafon BB, Rovina RL, Muñoz VR, da Silva LE, et al. Impact of different physical exercises on the expression of autophagy markers in mice. Int J Mol Sci. 2021;22(5):2635. [view at publisher] [DOI] [PMID] [Google Scholar]
44. Li Y, Zong W-X, Ding W-X. Recycling the danger via lipid droplet biogenesis after autophagy. Autophagy. 2017;13(11):1995-7. [view at publisher] [DOI] [PMID] [Google Scholar]
45. Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol. 2015;16(8):461-72. [view at publisher] [DOI] [PMID] [Google Scholar]
46. Zechner R, Madeo F, Kratky D. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat Rev Mol Cell Biol. 2017;18(11):671-84. [view at publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






