Volume 11, Issue 3 (12-2023)
Jorjani Biomed J 2023, 11(3): 9-13 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Koroni R, Zar A, Khaleghi M M. Effect of the type of exercise on lipid profile in menopausal women with type 2 diabetes: An interventional study in Iranian women. Jorjani Biomed J 2023; 11 (3) :9-13
URL: http://goums.ac.ir/jorjanijournal/article-1-982-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-982-en.html
1- Research Center of Persian Gulf Sports, Nutrition and Health, School of Literature and Humanities, Persian Gulf University, Boushehr, Iran , r.koroni1371@gmail.com
2- Department of Sport Science, School of Literature and Humanities, Persian Gulf University, Boushehr, Iran
2- Department of Sport Science, School of Literature and Humanities, Persian Gulf University, Boushehr, Iran
Keywords: Menopause, Diabetes Mellitus, Exercises, Cholesterol, Triglycerides, High-density lipoproteins
Full-Text [PDF 641 kb]
(2750 Downloads)
| Abstract (HTML) (9104 Views)
Full-Text: (1562 Views)
Introduction
Type 2 diabetes is characterized by a multitude of pathophysiological components, including insulin resistance, defective insulin secretion, adiposity, decreased incretin effect, increased glucagon secretion, and dyslipidemia (1). According to the International Diabetes Federation updates, an estimated 451 million people globally had type 2 diabetes mellitus (T2DM) in 2017, and this number is predicted to increase to 693 million by 2045, with a rise in prevalence from an estimated 8.4% to 9.9% (2). Type 2 diabetes mellitus accounts for more than 90% of diabetes cases and is associated with high morbidity and mortality (3). Diabetes is the leading cause of obesity (4). Type 2 diabetes is linked to obesity, hyperlipidemia, hypertension, elevated fasting blood glucose, and glycosylated hemoglobin, which, along with a lack of physical activity, increase the risk of cardiovascular disorders (5,6). Several risk factors elevate cardiovascular risk in patients with obesity and diabetes. Among these factors in diabetics are elevated circulating levels of triglycerides (TG), cholesterol, low-density lipoprotein (LDL), and reduced high-density lipoprotein (HDL) (7).
Current research shows that lifestyle interventions (e.g., diet and physical activity) may lead to diabetes remission among patients with T2DM (8). Additionally, physical activity is recommended for the prevention and treatment of diabetes (9). Different types of physical activity have different effects on the risk of diabetes, with a higher total level of physical activity associated with a significant reduction in diabetes risk (9). The American College of Sports Medicine (ACSM) has recommended physical activity and exercise as key strategies for managing T2DM (10). Physical activity may also be a technique for managing risk factors for cardiovascular diseases (CVD) in primary prevention. Training can reduce plasma LDL-C and TG concentrations while increasing HDL levels and the ratio of HDL to LDL (11).
Long-term aerobic exercise combined with resistance exercise has a greater impact on reducing inflammatory markers than aerobic or resistance training alone (12). Combined exercise involves aerobic exercise and resistance exercise performed within the same or separate exercise sessions of a training program. Compelling evidence shows that aerobic exercise has a positive effect on receptor affinity (adipose tissue, skeletal muscle, and insulin receptors), thereby promoting insulin sensitivity and glucose homeostasis. Resistance exercise can enhance muscle strength, insulin sensitivity, and muscle rehabilitation (13). Combined exercise (aerobic + resistance) is more effective in improving insulin resistance than aerobic or resistance training alone (14). On the other hand, Kambic et al. (2023) demonstrated that resistance training, when combined with aerobic training, had no additional effect beyond aerobic training alone on fasting glucose metabolism, blood lipids, and body composition in patients with coronary artery disease (CAD) (15). The results of the study showed a decrease in blood lipids, including TGs, cholesterol-HDL, and cholesterol-LDL, in the resistance group (16). Hakimi et al. (2015) observed a significant decrease in fat mass, fat percentage, cholesterol, TGs, and LDL in the exercise group (17).
Gorzi et al. (2012) demonstrated that performing combined exercises (aerobic and resistance) reduces blood sugar, LDL-cholesterol, and TG levels compared to each of these exercises individually (18). Given the varying research results in this field, the present study aimed to compare the effect of the type of exercise on the lipid profile in menopausal women with type 2 diabetes.
Methods
Subjects
Fifty-six sedentary obese menopausal women with type 2 diabetes, with a median age of 54.3 years (range: 48-61 years), were recruited for this study. The subjects were randomly divided into 4 groups: aerobic exercise (AE, n: 14), resistance exercise (RE, n: 14), combined exercise (CE, n: 13), and a control group (Co, n: 15). All subjects underwent comprehensive medical screening, including a medical history review, physical examination, and stress testing, before participation. Inclusion criteria included having type 2 diabetes according to the American Diabetes Association criteria, menopausal status, female sex, an age range of 48-61 years, and fasting blood sugar levels above 126 mg/dL. Exclusion criteria comprised smoking, a history of CAD, renal impairment or proteinuria, hepatic impairment, gout or hyperuricemia, uncontrolled hypertension (systolic blood pressure >160 mmHg), diabetic neuropathy, or retinopathy.
Ethical aspects
After the aim of the study and potential risks were explained, all subjects provided written informed consent. The protocol of this randomized controlled trial was conducted in accordance with the Declaration of Helsinki and was approved to comply with the Ethical Standards in Research of the Ministry of Science, Research, and Technology, with the code IR/SSRI.REC.2022.13742.1859.
Aerobic Exercise (AE) Protocol
Aerobic exercise consisted of running on the treadmill 3 times per week on non-consecutive days. In the group-based training sessions, the exercise time was gradually increased from 30 to 60 minutes in each session after 2 weeks. The exercise intensity was determined based on the maximum heart rate (HRmax), which was estimated for each participant using the following formula: HRmax = 208 - 0.7 × age. The training intensity started at 60% HRmax and was gradually increased to 75% HRmax during each session (Table 1). Heart rate was regularly monitored using the treadmill's built-in monitor (19).
Resistance Exercise (RE) Protocol
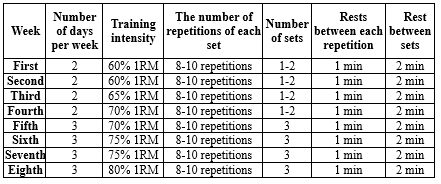
Resistance training consists of 3 sets of 8-10 repetitions with 1-minute rest between each repetition and 2 minutes of rest between sets for 8 weeks (20, 21) (Table 2).
Combined Exercise (CE) protocol
The combined exercise consisted of aerobic exercise plus strength training 3 times per week for 8 weeks. The subjects worked for 20-30 minutes on a treadmill plus 2 sets of each of 8 exercises with 8-10 repetitions on weight machines.
Control group
The control group had their daily activities and did not participate in any exercise program.
Laboratory measurements
Blood samples were collected before the start of the program (PRE) and 48 hours after the last exercise session (POST) from the antecubital vein through repetitive venous puncture while the participants were in a seated position. The blood was allowed to clot at room temperature and then centrifuged at 3500 rpm for 20 minutes to obtain the serum. The serum was carefully separated and stored at -70 °C for subsequent analysis of HDL, LDL, TG, and cholesterol levels. Serum levels of LDL were measured using the direct Chol-LDL method, HDL was measured using the HDL-Chol Direct method, and TG levels were measured using the lipase/PAP-GPO method, all of which were conducted using Pars Azmoun kits manufactured in Iran.
Anthropometric measurements
Anthropometric characteristics, such as height (cm), body weight (kg), body mass index (BMI), body fat percentage, and waist-to-hip ratio (WHR) measurements, were measured before (PRE) and 48 h after the program (POST).
Statistical analysis
To analyze the data, we employed analysis of variance (ANOVA) to assess significant differences between the groups before and after the training. Additionally, paired sample t tests were used for specific comparisons. All calculations were performed using SPSS v. 20.0 (SPSS, Chicago, IL). The results for all parameters are presented as mean values ± standard deviation (SD). The level of statistical significance was set at P-Value < 0.05.
Results
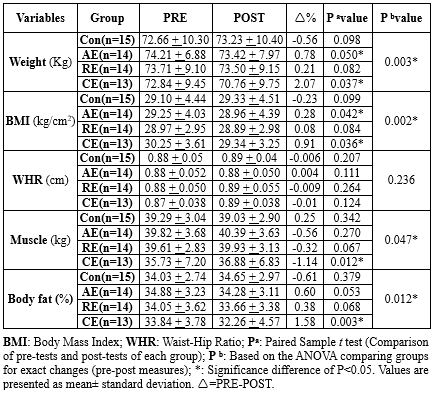
Body Weight, Muscle Weight, BMI, Body Fat Percentage
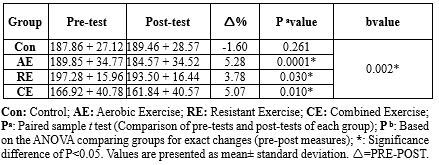
The group that engaged in a combination of aerobic and resistance exercises (2.86%) and the aerobic exercise group (1.06%) both showed a significant decrease in their body weight after 8 weeks of exercise (P < 0.05). In contrast, the control sedentary group and the resistance exercise group showed no significant change in their body weight (P > 0.05). Both aerobic (0.99%) and the combination of aerobic and resistance (3.01%) exercises led to a decrease in BMI, while other exercise interventions had no significant effect on BMI. The CE group also reduced body fat percentage by 4.67% (P < 0.05), whereas other exercise interventions had no significant effect on body fat percentage. When comparing the groups, a significantly greater reduction in BMI was observed in the CE group compared to the RE (0.91 to 0.084; P = 0.041) and control (0.91 to -0.23; P = 0.001) groups. Similarly, when comparing exercise groups to the control for body weight, body fat percentage, and muscle mass, the results showed percentage changes and corresponding P-values (Table 3).
LDL Levels
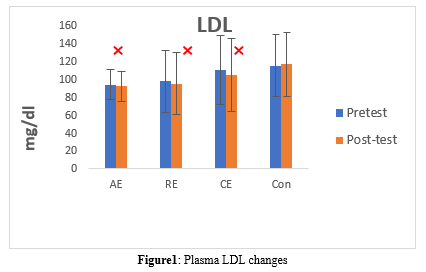
The LDL levels changed after an 8-week intervention in the Con, AE, RE, and CE groups (P = 0.001). In the Con group, the LDL level increased (pre: 114.82 ± 34.62 vs. post: 116.42 ± 35.78, P = 0.147, which is not significant). However, the LDL level significantly decreased in the AE (pre: 93.64 ± 16.86 vs. post: 91.57 ± 16.35, P = 0.034), RE (pre: 97.14 ± 34.37 vs. post: 94.71 ± 34.95, P = 0.003), and CE (pre: 109.69 ± 38.82 vs. post: 104.38 ± 41.09, P = 0.009) groups (Table 4, Figure 1).
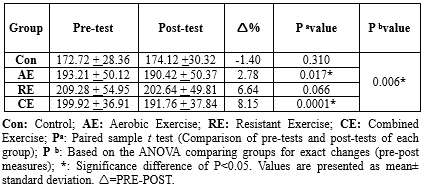
Triglyceride Levels
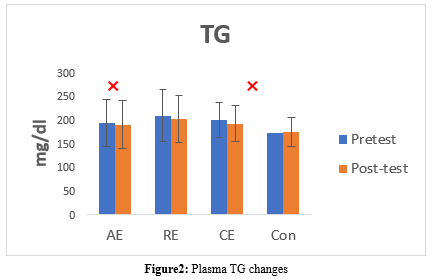
The TG level significantly changed after an 8-week intervention only in the AE and CE groups (P = 0.006). The TG level decreased significantly in the AE (pre: 193.21 ± 50.12 vs. post: 190.42 ± 50.37, P = 0.017) and CE (pre: 199.92 ± 36.91 vs. post: 191.76 ± 37.84, P = 0.0001) groups. No significant changes were detected in the Con (pre: 172.72 ± 28.36 vs. post: 174.12 ± 30.32, P = 0.310) and RE (pre: 209.28 ± 54.95 vs. post: 202.64 ± 49.81, P = 0.066) groups (Table 5, Figure 2).
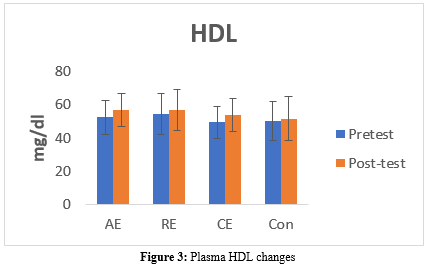
High-Density Lipoprotein Levels
The HDL level significantly increased in the AE (pre: 52.35 ± 10.42 vs. post: 56.92 ± 10.11, P = 0.041) and CE (pre: 49.61 ± 9.64 vs. post: 53.69 ± 9.87, P = 0.002) groups. However, there were no significant changes in the Con (pre: 50.13 ± 11.70 vs. post: 51.66 ± 13.16, P = 0.166) and RE (pre: 54.28 ± 12.28 vs. post: 56.64 ± 12.41, P = 0.117) groups (Table 6, Figure 3).
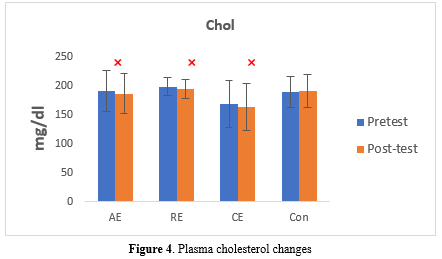
Total Cholesterol Levels
The total cholesterol (TC) levels significantly decreased in all the exercise intervention groups: AE (pre: 189.85 ± 34.77 vs. post: 184.57 ± 34.52, P = 0.041), RE (pre: 197.28 ± 15.96 vs. post: 193.50 ± 16.44, P = 0.030), and CE (pre: 166.92 ± 40.78 vs. post: 161.84 ± 40.57, P = 0.010). On the other hand, the Con group showed no significant change (pre: 187.86 ± 27.12 vs. post: 189.46 ± 28.57, P = 0.261) (Table 7, Figure 4).
Discussion
The present study aimed to compare the effect of different types of exercises on the lipid profile of menopausal women with type 2 diabetes. In this study, we observed a significant reduction in serum levels of LDL and cholesterol in all exercise groups. These findings align with the results of several previous studies (22-24). For instance, Rahimpour et al. (2022) demonstrated that short-term vitamin D supplementation had an impact on liver enzymes and lipid profile changes following endurance exercise in overweight women with non-alcoholic fatty liver disease (NAFLD), leading to a decrease in LDL and total serum cholesterol levels (22). Similarly, Du-Hwan Oh and Jang-Kyu Lee (2023) found that a combination of aerobic and resistance exercises significantly reduced TC and LDL levels in obese middle-aged women (23). Sadowska-Krępa et al. (2020) reported that a 12-week intervention involving Nordic walking exercise and a modified diet resulted in a notable reduction in TC and low-density lipoprotein cholesterol (LDL-C) among overweight and obese individuals who had previously worked in the coal mining industry (24).
However, some studies have reported results that are inconsistent with our findings (25,26). For instance, Liu et al. (2023) evaluated time-restricted nutrition and walking exercise interventions in female students with hidden obesity, and despite positive effects on the health of the participants, they observed an increase in TC levels (25). Additionally, Ratajczak et al. (2019) found that combined endurance and strength training in obese women had positive effects but led to an increase in cholesterol levels (26). Possible reasons for the discrepancies in research findings may include differences in age (24,25) sex (22,23) exercise type (25) duration (22-24), exercise intensity (22-25), and intervention protocols (22, 25).
It is worth noting that calcium can bind with bile acids, promoting their excretion in feces, which in turn contributes to a decrease in serum cholesterol levels. Consequently, vitamin D may potentially lower cholesterol, TG, and LDL levels by enhancing calcium absorption (27,28). Furthermore, elevated lipoprotein lipase activity resulting from increased physical activity enhances the catabolism of TG-rich lipoproteins, leading to a reduction in LDL levels (29).
Another significant finding of this study was a decrease in serum TG levels in the aerobic and combined exercise groups. These results are consistent with previous research findings (4,23,30). For instance, Du-Hwan Oh and Jang-Kyu Lee (2023) reported a substantial decrease in TG levels among middle-aged obese women who engaged in a combined regimen of aerobic and resistance exercises (23). Similarly, Costa et al. (2019) found that performing water-based aerobic exercises (WA) in dyslipidemic elderly women led to a reduction in TG levels (30). Additionally, Nazarieh et al. (2023) demonstrated that aerobic exercise in women with obesity and type 2 diabetes resulted in reduced TG levels (4).
However, the results of some studies were inconsistent with the findings of our study (31,32). For instance, Shakoor et al. (2023) found that engaging in moderate-intensity exercise led to an elevation in TG levels in male Wistar rats (31). Similarly, the results of Banz et al. (2003) showed that aerobic training for 10 weeks resulted in increased TG levels (32). Several factors may account for the discrepancies in research findings, including differences in age (30-32), sex (23,31,32), type of exercise (4,30-32), duration of exercise (23,30-32), exercise intensity (4,23,30-32), and other interventions (4,30,31).
Engaging in aerobic exercise has been shown to increase mitochondrial volume and the activity of lipolysis-effective enzymes. This enhancement contributes to an elevated capacity for fat catabolism during physical activity, consequently leading to a reduction in TG levels. While the specific mechanism behind the impact on HDL remains unclear, it is suggested that lecithin-cholesterol acetyltransferase (L-CAT) and hepatic lipase (HL) may play a role in facilitating HDL's function in the reverse transport of cholesterol from arterial walls (33).
Another significant finding of this study was a significant increase in serum HDL levels in the aerobic and combination exercise groups compared to the resistance training group. These results are consistent with those of several previous studies (26,34,35). For example, Ratajczak et al. (2019) reported a significant increase in high-density lipoprotein cholesterol (HDL-C) concentration among obese women subjected to endurance training (26). Similarly, the results of Said et al.'s research (2021) indicated that HDL-C concentration increased in the studied samples that engaged in resistance and aerobic exercise (34). Ozbay et al.'s study (2020) demonstrated that both indoor and outdoor aerobic exercises conducted during the winter resulted in an increase in HDL-C levels (35).
However, the results of some studies were inconsistent with the findings of our study (36). For instance, Streb et al. (2022) found that engaging in combined exercise among obese adults led to a reduction in the levels of HDL (36). Possible reasons for the lack of consistency in research findings include differences in age (26,34-36), sex (26,34-36), type of exercise (26,34-36), duration of exercise (26,34-36), exercise intensity (26,34-36), and seasonal variations (35).
Consequently, the improvement in the oxidant-antioxidant balance in both blood and tissues, as a result of physical training, appears to be a potential mechanism contributing to the systemic regulation of endothelial function. Elevated levels of malondialdehyde (MDA) in the blood lead to its binding with HDL-C particles. This binding subsequently leads to the formation of modified HDL-C lipoproteins, which then activate protein kinase C-β2 (PKC-β2). Activation of PKC-β2 results in decreased phosphorylation of endothelial nitric oxide synthase (eNOS), thereby reducing the release of nitric oxide (NO) by the endothelium (37). Schuler et al. (38) suggest that alterations in HDL-C metabolism during exercise may occur, in part, to sequester elevated concentrations of MDA in the blood.
Conclusion
Overall, this study demonstrated the effectiveness of all three training methods in improving cardiovascular risk factors and lipid profiles in menopausal women with diabetes. However, combined exercises, in addition to providing greater resistance than aerobic or resistance training alone, proved to be particularly effective in reducing risk factors for cardiovascular disease in menopausal women with type 2 diabetes. It is considered the most effective type of exercise for improving cardiovascular risk factors and lipid profiles. Additionally, combined exercises resulted in significant changes in body composition, including BMI and body fat percentage, in menopausal women with diabetes.
Limitations of the study
The psychological condition of the subjects and the lack of accurate control of their nutrition (nutrition control through the material registration sheet) are the limitations of the present study.
Acknowledgement
The authors would like to thank the subjects for their committed participation.
Funding sources
This study did not receive any funds.
Ethical statement
The protocol of this randomized controlled trial was administered in accordance with the Declaration of Helsinki and was approved according to compliance with Ethical Standards in Research of the Ministry of Science, Research and Technology, with the code IR/SSRI.REC.2022.13742.1859.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
RK: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - editing, Visualization, Project administration, Formal analysis, Funding acquisition. AZ: Writing- Original draft preparation, Writing – editing. MMK: Writing- Original draft preparation.
Type 2 diabetes is characterized by a multitude of pathophysiological components, including insulin resistance, defective insulin secretion, adiposity, decreased incretin effect, increased glucagon secretion, and dyslipidemia (1). According to the International Diabetes Federation updates, an estimated 451 million people globally had type 2 diabetes mellitus (T2DM) in 2017, and this number is predicted to increase to 693 million by 2045, with a rise in prevalence from an estimated 8.4% to 9.9% (2). Type 2 diabetes mellitus accounts for more than 90% of diabetes cases and is associated with high morbidity and mortality (3). Diabetes is the leading cause of obesity (4). Type 2 diabetes is linked to obesity, hyperlipidemia, hypertension, elevated fasting blood glucose, and glycosylated hemoglobin, which, along with a lack of physical activity, increase the risk of cardiovascular disorders (5,6). Several risk factors elevate cardiovascular risk in patients with obesity and diabetes. Among these factors in diabetics are elevated circulating levels of triglycerides (TG), cholesterol, low-density lipoprotein (LDL), and reduced high-density lipoprotein (HDL) (7).
Current research shows that lifestyle interventions (e.g., diet and physical activity) may lead to diabetes remission among patients with T2DM (8). Additionally, physical activity is recommended for the prevention and treatment of diabetes (9). Different types of physical activity have different effects on the risk of diabetes, with a higher total level of physical activity associated with a significant reduction in diabetes risk (9). The American College of Sports Medicine (ACSM) has recommended physical activity and exercise as key strategies for managing T2DM (10). Physical activity may also be a technique for managing risk factors for cardiovascular diseases (CVD) in primary prevention. Training can reduce plasma LDL-C and TG concentrations while increasing HDL levels and the ratio of HDL to LDL (11).
Long-term aerobic exercise combined with resistance exercise has a greater impact on reducing inflammatory markers than aerobic or resistance training alone (12). Combined exercise involves aerobic exercise and resistance exercise performed within the same or separate exercise sessions of a training program. Compelling evidence shows that aerobic exercise has a positive effect on receptor affinity (adipose tissue, skeletal muscle, and insulin receptors), thereby promoting insulin sensitivity and glucose homeostasis. Resistance exercise can enhance muscle strength, insulin sensitivity, and muscle rehabilitation (13). Combined exercise (aerobic + resistance) is more effective in improving insulin resistance than aerobic or resistance training alone (14). On the other hand, Kambic et al. (2023) demonstrated that resistance training, when combined with aerobic training, had no additional effect beyond aerobic training alone on fasting glucose metabolism, blood lipids, and body composition in patients with coronary artery disease (CAD) (15). The results of the study showed a decrease in blood lipids, including TGs, cholesterol-HDL, and cholesterol-LDL, in the resistance group (16). Hakimi et al. (2015) observed a significant decrease in fat mass, fat percentage, cholesterol, TGs, and LDL in the exercise group (17).
Gorzi et al. (2012) demonstrated that performing combined exercises (aerobic and resistance) reduces blood sugar, LDL-cholesterol, and TG levels compared to each of these exercises individually (18). Given the varying research results in this field, the present study aimed to compare the effect of the type of exercise on the lipid profile in menopausal women with type 2 diabetes.
Methods
Subjects
Fifty-six sedentary obese menopausal women with type 2 diabetes, with a median age of 54.3 years (range: 48-61 years), were recruited for this study. The subjects were randomly divided into 4 groups: aerobic exercise (AE, n: 14), resistance exercise (RE, n: 14), combined exercise (CE, n: 13), and a control group (Co, n: 15). All subjects underwent comprehensive medical screening, including a medical history review, physical examination, and stress testing, before participation. Inclusion criteria included having type 2 diabetes according to the American Diabetes Association criteria, menopausal status, female sex, an age range of 48-61 years, and fasting blood sugar levels above 126 mg/dL. Exclusion criteria comprised smoking, a history of CAD, renal impairment or proteinuria, hepatic impairment, gout or hyperuricemia, uncontrolled hypertension (systolic blood pressure >160 mmHg), diabetic neuropathy, or retinopathy.
Ethical aspects
After the aim of the study and potential risks were explained, all subjects provided written informed consent. The protocol of this randomized controlled trial was conducted in accordance with the Declaration of Helsinki and was approved to comply with the Ethical Standards in Research of the Ministry of Science, Research, and Technology, with the code IR/SSRI.REC.2022.13742.1859.
Aerobic Exercise (AE) Protocol
Aerobic exercise consisted of running on the treadmill 3 times per week on non-consecutive days. In the group-based training sessions, the exercise time was gradually increased from 30 to 60 minutes in each session after 2 weeks. The exercise intensity was determined based on the maximum heart rate (HRmax), which was estimated for each participant using the following formula: HRmax = 208 - 0.7 × age. The training intensity started at 60% HRmax and was gradually increased to 75% HRmax during each session (Table 1). Heart rate was regularly monitored using the treadmill's built-in monitor (19).
|
Table 1. Aerobic exercise protocol
 |
Resistance training consists of 3 sets of 8-10 repetitions with 1-minute rest between each repetition and 2 minutes of rest between sets for 8 weeks (20, 21) (Table 2).
- The protocol started on 2 days per week during the first month and was increased to 3 days per week.
- The training started during weeks 1 and 2 with a 60% 1-repetition maximum (1RM) and then increased to 75-80% 1RM.
- The number of sets was 1-2 during the first month; then, it was increased to 3 sets during the second month.
- This program included 10 different exercises for the upper and lower body, such as bench press, seated row, shoulder press, chest press, lateral pulldown, abdominal crunches, leg press, leg extension, triceps pushdown, and seated bicep curls.
The combined exercise consisted of aerobic exercise plus strength training 3 times per week for 8 weeks. The subjects worked for 20-30 minutes on a treadmill plus 2 sets of each of 8 exercises with 8-10 repetitions on weight machines.
Control group
The control group had their daily activities and did not participate in any exercise program.
Laboratory measurements
Blood samples were collected before the start of the program (PRE) and 48 hours after the last exercise session (POST) from the antecubital vein through repetitive venous puncture while the participants were in a seated position. The blood was allowed to clot at room temperature and then centrifuged at 3500 rpm for 20 minutes to obtain the serum. The serum was carefully separated and stored at -70 °C for subsequent analysis of HDL, LDL, TG, and cholesterol levels. Serum levels of LDL were measured using the direct Chol-LDL method, HDL was measured using the HDL-Chol Direct method, and TG levels were measured using the lipase/PAP-GPO method, all of which were conducted using Pars Azmoun kits manufactured in Iran.
Anthropometric measurements
Anthropometric characteristics, such as height (cm), body weight (kg), body mass index (BMI), body fat percentage, and waist-to-hip ratio (WHR) measurements, were measured before (PRE) and 48 h after the program (POST).
Statistical analysis
To analyze the data, we employed analysis of variance (ANOVA) to assess significant differences between the groups before and after the training. Additionally, paired sample t tests were used for specific comparisons. All calculations were performed using SPSS v. 20.0 (SPSS, Chicago, IL). The results for all parameters are presented as mean values ± standard deviation (SD). The level of statistical significance was set at P-Value < 0.05.
Results
Body Weight, Muscle Weight, BMI, Body Fat Percentage
The group that engaged in a combination of aerobic and resistance exercises (2.86%) and the aerobic exercise group (1.06%) both showed a significant decrease in their body weight after 8 weeks of exercise (P < 0.05). In contrast, the control sedentary group and the resistance exercise group showed no significant change in their body weight (P > 0.05). Both aerobic (0.99%) and the combination of aerobic and resistance (3.01%) exercises led to a decrease in BMI, while other exercise interventions had no significant effect on BMI. The CE group also reduced body fat percentage by 4.67% (P < 0.05), whereas other exercise interventions had no significant effect on body fat percentage. When comparing the groups, a significantly greater reduction in BMI was observed in the CE group compared to the RE (0.91 to 0.084; P = 0.041) and control (0.91 to -0.23; P = 0.001) groups. Similarly, when comparing exercise groups to the control for body weight, body fat percentage, and muscle mass, the results showed percentage changes and corresponding P-values (Table 3).
|
Table 3. Subject characteristics in the groups at baseline and after the 8-week intervention
 |
The LDL levels changed after an 8-week intervention in the Con, AE, RE, and CE groups (P = 0.001). In the Con group, the LDL level increased (pre: 114.82 ± 34.62 vs. post: 116.42 ± 35.78, P = 0.147, which is not significant). However, the LDL level significantly decreased in the AE (pre: 93.64 ± 16.86 vs. post: 91.57 ± 16.35, P = 0.034), RE (pre: 97.14 ± 34.37 vs. post: 94.71 ± 34.95, P = 0.003), and CE (pre: 109.69 ± 38.82 vs. post: 104.38 ± 41.09, P = 0.009) groups (Table 4, Figure 1).

Triglyceride Levels
The TG level significantly changed after an 8-week intervention only in the AE and CE groups (P = 0.006). The TG level decreased significantly in the AE (pre: 193.21 ± 50.12 vs. post: 190.42 ± 50.37, P = 0.017) and CE (pre: 199.92 ± 36.91 vs. post: 191.76 ± 37.84, P = 0.0001) groups. No significant changes were detected in the Con (pre: 172.72 ± 28.36 vs. post: 174.12 ± 30.32, P = 0.310) and RE (pre: 209.28 ± 54.95 vs. post: 202.64 ± 49.81, P = 0.066) groups (Table 5, Figure 2).
|
Table 5. Triglyceride value in the groups at baseline and after the 8-week intervention
 |

High-Density Lipoprotein Levels
The HDL level significantly increased in the AE (pre: 52.35 ± 10.42 vs. post: 56.92 ± 10.11, P = 0.041) and CE (pre: 49.61 ± 9.64 vs. post: 53.69 ± 9.87, P = 0.002) groups. However, there were no significant changes in the Con (pre: 50.13 ± 11.70 vs. post: 51.66 ± 13.16, P = 0.166) and RE (pre: 54.28 ± 12.28 vs. post: 56.64 ± 12.41, P = 0.117) groups (Table 6, Figure 3).
|
Table 6. High-Density lipoprotein value in the groups at baseline and after the 8-week intervention
 |

Total Cholesterol Levels
The total cholesterol (TC) levels significantly decreased in all the exercise intervention groups: AE (pre: 189.85 ± 34.77 vs. post: 184.57 ± 34.52, P = 0.041), RE (pre: 197.28 ± 15.96 vs. post: 193.50 ± 16.44, P = 0.030), and CE (pre: 166.92 ± 40.78 vs. post: 161.84 ± 40.57, P = 0.010). On the other hand, the Con group showed no significant change (pre: 187.86 ± 27.12 vs. post: 189.46 ± 28.57, P = 0.261) (Table 7, Figure 4).

Discussion
The present study aimed to compare the effect of different types of exercises on the lipid profile of menopausal women with type 2 diabetes. In this study, we observed a significant reduction in serum levels of LDL and cholesterol in all exercise groups. These findings align with the results of several previous studies (22-24). For instance, Rahimpour et al. (2022) demonstrated that short-term vitamin D supplementation had an impact on liver enzymes and lipid profile changes following endurance exercise in overweight women with non-alcoholic fatty liver disease (NAFLD), leading to a decrease in LDL and total serum cholesterol levels (22). Similarly, Du-Hwan Oh and Jang-Kyu Lee (2023) found that a combination of aerobic and resistance exercises significantly reduced TC and LDL levels in obese middle-aged women (23). Sadowska-Krępa et al. (2020) reported that a 12-week intervention involving Nordic walking exercise and a modified diet resulted in a notable reduction in TC and low-density lipoprotein cholesterol (LDL-C) among overweight and obese individuals who had previously worked in the coal mining industry (24).
However, some studies have reported results that are inconsistent with our findings (25,26). For instance, Liu et al. (2023) evaluated time-restricted nutrition and walking exercise interventions in female students with hidden obesity, and despite positive effects on the health of the participants, they observed an increase in TC levels (25). Additionally, Ratajczak et al. (2019) found that combined endurance and strength training in obese women had positive effects but led to an increase in cholesterol levels (26). Possible reasons for the discrepancies in research findings may include differences in age (24,25) sex (22,23) exercise type (25) duration (22-24), exercise intensity (22-25), and intervention protocols (22, 25).
It is worth noting that calcium can bind with bile acids, promoting their excretion in feces, which in turn contributes to a decrease in serum cholesterol levels. Consequently, vitamin D may potentially lower cholesterol, TG, and LDL levels by enhancing calcium absorption (27,28). Furthermore, elevated lipoprotein lipase activity resulting from increased physical activity enhances the catabolism of TG-rich lipoproteins, leading to a reduction in LDL levels (29).
Another significant finding of this study was a decrease in serum TG levels in the aerobic and combined exercise groups. These results are consistent with previous research findings (4,23,30). For instance, Du-Hwan Oh and Jang-Kyu Lee (2023) reported a substantial decrease in TG levels among middle-aged obese women who engaged in a combined regimen of aerobic and resistance exercises (23). Similarly, Costa et al. (2019) found that performing water-based aerobic exercises (WA) in dyslipidemic elderly women led to a reduction in TG levels (30). Additionally, Nazarieh et al. (2023) demonstrated that aerobic exercise in women with obesity and type 2 diabetes resulted in reduced TG levels (4).
However, the results of some studies were inconsistent with the findings of our study (31,32). For instance, Shakoor et al. (2023) found that engaging in moderate-intensity exercise led to an elevation in TG levels in male Wistar rats (31). Similarly, the results of Banz et al. (2003) showed that aerobic training for 10 weeks resulted in increased TG levels (32). Several factors may account for the discrepancies in research findings, including differences in age (30-32), sex (23,31,32), type of exercise (4,30-32), duration of exercise (23,30-32), exercise intensity (4,23,30-32), and other interventions (4,30,31).
Engaging in aerobic exercise has been shown to increase mitochondrial volume and the activity of lipolysis-effective enzymes. This enhancement contributes to an elevated capacity for fat catabolism during physical activity, consequently leading to a reduction in TG levels. While the specific mechanism behind the impact on HDL remains unclear, it is suggested that lecithin-cholesterol acetyltransferase (L-CAT) and hepatic lipase (HL) may play a role in facilitating HDL's function in the reverse transport of cholesterol from arterial walls (33).
Another significant finding of this study was a significant increase in serum HDL levels in the aerobic and combination exercise groups compared to the resistance training group. These results are consistent with those of several previous studies (26,34,35). For example, Ratajczak et al. (2019) reported a significant increase in high-density lipoprotein cholesterol (HDL-C) concentration among obese women subjected to endurance training (26). Similarly, the results of Said et al.'s research (2021) indicated that HDL-C concentration increased in the studied samples that engaged in resistance and aerobic exercise (34). Ozbay et al.'s study (2020) demonstrated that both indoor and outdoor aerobic exercises conducted during the winter resulted in an increase in HDL-C levels (35).
However, the results of some studies were inconsistent with the findings of our study (36). For instance, Streb et al. (2022) found that engaging in combined exercise among obese adults led to a reduction in the levels of HDL (36). Possible reasons for the lack of consistency in research findings include differences in age (26,34-36), sex (26,34-36), type of exercise (26,34-36), duration of exercise (26,34-36), exercise intensity (26,34-36), and seasonal variations (35).
Consequently, the improvement in the oxidant-antioxidant balance in both blood and tissues, as a result of physical training, appears to be a potential mechanism contributing to the systemic regulation of endothelial function. Elevated levels of malondialdehyde (MDA) in the blood lead to its binding with HDL-C particles. This binding subsequently leads to the formation of modified HDL-C lipoproteins, which then activate protein kinase C-β2 (PKC-β2). Activation of PKC-β2 results in decreased phosphorylation of endothelial nitric oxide synthase (eNOS), thereby reducing the release of nitric oxide (NO) by the endothelium (37). Schuler et al. (38) suggest that alterations in HDL-C metabolism during exercise may occur, in part, to sequester elevated concentrations of MDA in the blood.
Conclusion
Overall, this study demonstrated the effectiveness of all three training methods in improving cardiovascular risk factors and lipid profiles in menopausal women with diabetes. However, combined exercises, in addition to providing greater resistance than aerobic or resistance training alone, proved to be particularly effective in reducing risk factors for cardiovascular disease in menopausal women with type 2 diabetes. It is considered the most effective type of exercise for improving cardiovascular risk factors and lipid profiles. Additionally, combined exercises resulted in significant changes in body composition, including BMI and body fat percentage, in menopausal women with diabetes.
Limitations of the study
The psychological condition of the subjects and the lack of accurate control of their nutrition (nutrition control through the material registration sheet) are the limitations of the present study.
Acknowledgement
The authors would like to thank the subjects for their committed participation.
Funding sources
This study did not receive any funds.
Ethical statement
The protocol of this randomized controlled trial was administered in accordance with the Declaration of Helsinki and was approved according to compliance with Ethical Standards in Research of the Ministry of Science, Research and Technology, with the code IR/SSRI.REC.2022.13742.1859.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
RK: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - editing, Visualization, Project administration, Formal analysis, Funding acquisition. AZ: Writing- Original draft preparation, Writing – editing. MMK: Writing- Original draft preparation.
Type of Article: Original article |
Subject:
Health
Received: 2023/08/14 | Accepted: 2023/12/12 | Published: 2023/12/20
Received: 2023/08/14 | Accepted: 2023/12/12 | Published: 2023/12/20
References
1. De Block C, Bailey C, Wysham C, Hemmingway A, Allen SE, Peleshok J. Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective. Diabetes Obes Metab. 2023;25(1):3-17. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Zaki S, Sharma S, Vats H. Effectiveness of concurrent exercise training in people with type 2 diabetes: A systematic review and meta-analysis. Physiother Theory Pract. 2023:1-22. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Amin M, Kerr D, Atiase Y, Aldwikat RK, Driscoll A. Effect of Physical Activity on Metabolic Syndrome Markers in Adults with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Sports. 2023;11(5):101. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Nazarieh S, Aminaei M, Nikoei R. The Effect of Aerobic Exercise and Consumption of Eryngium Billardieri Extract On Women with Obesity and Type 2 Diabetes. J Nutr Fast Health. 2023;11(1):15-23. [View at Publisher] [DOI] [Google Scholar]
5. Nathan DM, Group DER. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes care. 2014;37(1):9-16. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin diabetes. 2008;26(2):77-82. [View at Publisher] [DOI] [Google Scholar]
7. Yousefipoor P, Tadibi V, Behpoor N, Parnow A, Dalbari M, Rashidi S. Effects of aerobic exercise on glycemic control and risk factors CVD in people with type 2 diabetes. Med J Mashhad Univ Med Sci. 2015;57(9):976-84. [View at Publisher] [DOI] [Google Scholar]
8. Zhang Y, Yang Y, Huang Q, Zhang Q, Li M, Wu Y. The effectiveness of lifestyle interventions for diabetes remission on patients with type 2 diabetes mellitus: A systematic review and meta‐analysis. Worldviews Evid Based Nurs. 2023;20(1):64-78. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Li C, Shang S, Liang W. Physical Activity Types, Physical Activity Levels and Risk of Diabetes in General Adults: The NHANES 2007-2018. Int J Environ Res Public Health. 2023;20(2):1398. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Kanaley JA, Colberg SR, Corcoran MH, Malin SK, Rodriguez NR, Crespo CJ, et al. Exercise/physical activity in individuals with type 2 diabetes: a consensus statement from the American College of Sports Medicine. Med Sci Sports Exerc. 2022;54(2):353-68. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Doewes RI, Gharibian G, Abolhasani Zadeh F, Zaman BA, Vahdat S, Akhavan-Sigari R. An updated systematic review on the effects of aerobic exercise on human blood lipid profile. Curr Probl Cardiol. 2023;48(5):101-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Hopps E, Canino B, Caimi G. Effects of exercise on inflammation markers in type 2 diabetic subjects. Acta Diabetol. 2011;48(3):183-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Zhao X, He Q, Zeng Y, Cheng L. Effectiveness of combined exercise in people with type 2 diabetes and concurrent overweight/obesity: A systematic review and meta-analysis. BMJ Open. 2021;11(10):e046252. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Sigal RJ, Kenny GP. Combined aerobic and resistance exercise for patients with type 2 diabetes. JAMA. 2010;304(20):2298-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Kambic T, Božič Mijovski M, Jug B, Hadžić V, Lainscak M. Insulin resistance, lipids and body composition in patients with coronary artery disease after combined aerobic training and resistance training: a randomised, controlled trial. Diabetol Metab Syndr. 2023;15(1):47. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Jorge MLMP, de Oliveira VN, Resende NM, Paraiso LF, Calixto A, Diniz ALD, et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. 2011;60(9):1244-52. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Hakimi M, Sheikholeslami-Vatani D, Ali-Mohammadi M. Effect of Concurrent Training with ingested of L-carnitine supplementation on hormonal changes, lipid profile and body composition in obese men. Studies in Medical Sciences. 2015;26(3):185-93. [View at Publisher] [Google Scholar]
18. Gorzi A, Rajabi H, Azad A, molanouri shamsi M, Hedayati M. Effect of concurrent, strength and endurance training on hormones, lipids and inflammatory characteristics of untrained men. Iran J Endocrinol Metabol. 2012;13(6):614-20. [View at Publisher] [Google Scholar]
19. Amanat S, Sinaei E, Panji M, MohammadporHodki R, Bagheri-Hosseinabadi Z, Asadimehr H, et al. A randomized controlled trial on the effects of 12 weeks of aerobic, resistance, and combined exercises training on the serum levels of nesfatin-1, irisin-1 and HOMA-IR. Front Physiol. 2020;11:562895. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Dehghan F, Hajiaghaalipour F, Yusof A, Muniandy S, Hosseini SA, Heydari S, et al. Saffron with resistance exercise improves diabetic parameters through the GLUT4/AMPK pathway in-vitro and in-vivo. Sci Rep. 2016;6(1):25139. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Durstine JL, Grandjean PW, Cox CA, Thompson PD. Lipids, lipoproteins, and exercise. J Cardiopulm Rehabil. 2002;22(6):385-98. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Rahimpour Z, Hoseini R, Behpour N. Alterations of liver enzymes and lipid profile in response to exhaustive eccentric exercise: vitamin D supplementation trial in overweight females with non-alcoholic fatty liver disease. BMC Gastroenterol. 2022;22(1):372. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Oh DH, Lee JK. Effect of Different Intensities of Aerobic Exercise Combined with Resistance Exercise on Body Fat, Lipid Profiles, and Adipokines in Middle-Aged Women with Obesity. Int J Environ Res Public Health. 2023;20(5):3991. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Sadowska-Krępa E, Gdańska A, Rozpara M, Pilch W, Přidalová M, Bańkowski S. Effect of 12-Week Interventions Involving Nordic Walking Exercise and a Modified Diet on the Anthropometric Parameters and Blood Lipid Profiles in Overweight and Obese Ex-Coal Miners. Obes Facts. 2020;13(2):201-12. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Liu H, Chen S, Ji H, Dai Z. Effects of time-restricted feeding and walking exercise on the physical health of female college students with hidden obesity: a randomized trial. Front Public Health. 2023;11:1020887. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Ratajczak M, Skrypnik D, Bogdański P, Mądry E, Walkowiak J, Szulińska M, et al. Effects of Endurance and Endurance-Strength Training on Endothelial Function in Women with Obesity: A Randomized Trial. Int J Environ Res Public Health. 2019;16(21):4291. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Carmeliet G, Dermauw V, Bouillon R. Vitamin D signaling in calcium and bone homeostasis: a delicate balance. Best Pract Res Clin Endocrinol Metab. 2015;29(4):621-31. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Oda Y, Hu L, Nguyen T, Fong C, Tu C-l, Bikle DD. Combined deletion of the vitamin D receptor and calcium-sensing receptor delays wound re-epithelialization. Endocrinology. 2017;158(6):1929-38. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Lennon SL, Quindry J, Hamilton KL, French J, Staib J, Mehta JL, et al. Loss of exercise-induced cardioprotection after cessation of exercise. J Appl Physiol. 2004;96(4):1299-305. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Costa RR, Buttelli ACK, Coconcelli L, Pereira LF, Vieira AF, Fagundes AO, et al. Water-Based Aerobic and Resistance Training as a Treatment to Improve the Lipid Profile of Women With Dyslipidemia: A Randomized Controlled Trial. J Phys Act Health. 2019;16(5):348-54. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Shakoor H, Kizhakkayil J, Khalid M, Mahgoub A, Platat C. Effect of Moderate-Intense Training and Detraining on Glucose Metabolism, Lipid Profile, and Liver Enzymes in Male Wistar Rats: A Preclinical Randomized Study. Nutrients. 2023;15(17):3820. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Banz WJ, Maher MA, Thompson WG, Bassett DR, Moore W, Ashraf M, et al. Effects of resistance versus aerobic training on coronary artery disease risk factors. Exp Biol Med (Maywood). 2003;228(4):434-40. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Plowman SA, Smith DL. Exercise physiology for health fitness and performance. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. [View at Publisher] [Google Scholar]
34. Said MA, Abdelmoneim MA, Alibrahim MS, Kotb AAH. Aerobic training, resistance training, or their combination as a means to fight against excess weight and metabolic syndrome in obese students - which is the most effective modality? A randomized controlled trial. Appl Physiol Nutr Metab. 2021;46(8):952-63. [View at Publisher] [DOI] [PMID] [Google Scholar]
35. Ozbay S, Ulupınar S, Şebin E, Altınkaynak K. Acute and chronic effects of aerobic exercise on serum irisin, adropin, and cholesterol levels in the winter season: Indoor training versus outdoor training. Chin J Physiol. 2020;63(1):21-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
36. Streb AR, Braga PGS, de Melo RF, Botelho LJ, Maranhão RC, Del Duca GF. Effects of combined physical exercise on plasma lipid variables, paraoxonase 1 activity, and inflammation parameters in adults with obesity: a randomized clinical trial. J Endocrinol Invest. 2022;45(10):1991-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
37. Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121(7):2693-708. [View at Publisher] [DOI] [PMID] [Google Scholar]
38. Schuler G, Adams V, Goto Y. Role of exercise in the prevention of cardiovascular disease: results, mechanisms, and new perspectives. Eur Heart J. 2013;34(24):1790-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |