Volume 11, Issue 3 (12-2023)
Jorjani Biomed J 2023, 11(3): 25-29 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zolfi H R, Shakib A, Niknam Z, pashaei Z. Impact of high-intensity interval training on Mir-21 and Mir-122 expression and metabolic parameters in middle-aged men with metabolic syndrome. Jorjani Biomed J 2023; 11 (3) :25-29
URL: http://goums.ac.ir/jorjanijournal/article-1-975-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-975-en.html
1- Department of Physical Education and Sport Science, Technical and Vocational University (TVU), Tehran, Iran
2- Faculty of Physical Education and Sport Sciences, University of Tabriz, Tabriz, Iran
3- Faculty of Physical Education and Sport Sciences, University of Tabriz, Tabriz, Iran ,pashaei.zh@gmail.com
2- Faculty of Physical Education and Sport Sciences, University of Tabriz, Tabriz, Iran
3- Faculty of Physical Education and Sport Sciences, University of Tabriz, Tabriz, Iran ,
Keywords: High-intensity interval training, MIRN21 microRNA, human, MIRN122 microRNA, human, Metabolic syndrome
Full-Text [PDF 520 kb]
(4417 Downloads)
| Abstract (HTML) (11935 Views)
Full-Text: (2109 Views)
Introduction
Metabolic syndrome (MetS) is a combination of several medical problems (1); according to studies, the prevalence of MetS is 15% in the European population and 27% in the American population (2). According to the available data, about one-third of the adult population in Iran (33.7%) suffer from MetS (3). Metabolic syndrome is a combination of several characteristics, including glucose intolerance, dyslipidemia (including hypertriglyceridemia), increased free fatty acid (FFA), decreased high-density lipoprotein (HDL), microalbuminuria, hypertension, nonalcoholic fatty liver disease (NAFLD), polycystic ovarian syndrome (PCOS), and oxidative stress. Obesity (especially abdominal adiposity) is the most significant risk factor in metabolic diseases; it is correlated with a wide cluster of metabolic disorders, including chronic low-grade inflammatory disease resulting in insulin resistance (IR) and type 2 diabetes mellitus (T2DM) (4-6). Considering the risk factors mentioned about MetS and the close relationship between the prevalence of obesity and a sedentary lifestyle, it is clearly shown that this syndrome has become a global health challenge (4, 7).
The health-related, preventive, and therapeutic effects of exercise training on metabolic disorders have been shown in different subjects (8, 9). High-intensity interval training (HIIT) is an excellent strategy to reduce metabolic disorders (10). It involves alternating short periods of intense or explosive anaerobic exercises separated by recovery periods (11). Despite confirming the positive effect of exercise training in preventing the onset of type 2 diabetes (T2D) and MetS, the molecular mechanisms involved in this field are completely unknown (7). It is known that miRNAs (microribonucleic acid) are involved in intercellular interactions; therefore, changes in the expression levels of miRNAs may reflect the details of changes in response to exercise. Thus, there is an urgent need to evaluate the response and function of new intercellular mediators, such as circulating miRNAs (12).
miRNAs (19-23 nucleotides) are single-stranded noncoding RNA molecules that play a role in the post-transcriptional control of the gene expression. miRNAs act as regulators of mRNA degradation and/or blocking protein translation through binding to their corresponding sequences within the 3’-untranslated regions (3'UTRs) (13). It has been manifested that miRNA, particularly serum miRNAs, are stable in blood circulation since they are resistant to RNase digestion. This shows great promise in becoming a novel diagnostic biomarker of MetS and its components (4, 6, 12, 14, 15). In the meantime, it has been shown that miR-21 and miR-122 are involved in glucose and lipid profile metabolism, liver disorders, and related physiological processes; thus, it has been suggested that interventions used to modulate these miRNAs can be a promising approach for treating metabolic diseases (16, 17). Kalaki-Jouybari et al. showed that HIIT is the best training method to improve the condition of NAFLD by inducing miR-122 compared to continuous training (18). After chronic endurance training, a decrease in the level of miR-21 in human plasma was observed (19). Also, the favorable effects of HIIT on alanine aminotransferase (ALT), aspartate aminotransferase (AST), lipid profile, and glucose metabolism have been shown (20, 21). However, it is still not clear whether HIIT can improve liver enzymes, glucose metabolic status, and lipid profile in patients with MetS by affecting the miR-122 and miR-21. Therefore, the effect of physical training on miR-122 and miR-21 in patients with MetS is unclear. Thus, this study aimed to explore the effect of HIIT training on changes in miR-21, miR-122, liver factors, lipid, and glucose factors in middle-aged men with MetS.
Methods
Participants
Metabolic syndrome (MetS) is a combination of several medical problems (1); according to studies, the prevalence of MetS is 15% in the European population and 27% in the American population (2). According to the available data, about one-third of the adult population in Iran (33.7%) suffer from MetS (3). Metabolic syndrome is a combination of several characteristics, including glucose intolerance, dyslipidemia (including hypertriglyceridemia), increased free fatty acid (FFA), decreased high-density lipoprotein (HDL), microalbuminuria, hypertension, nonalcoholic fatty liver disease (NAFLD), polycystic ovarian syndrome (PCOS), and oxidative stress. Obesity (especially abdominal adiposity) is the most significant risk factor in metabolic diseases; it is correlated with a wide cluster of metabolic disorders, including chronic low-grade inflammatory disease resulting in insulin resistance (IR) and type 2 diabetes mellitus (T2DM) (4-6). Considering the risk factors mentioned about MetS and the close relationship between the prevalence of obesity and a sedentary lifestyle, it is clearly shown that this syndrome has become a global health challenge (4, 7).
The health-related, preventive, and therapeutic effects of exercise training on metabolic disorders have been shown in different subjects (8, 9). High-intensity interval training (HIIT) is an excellent strategy to reduce metabolic disorders (10). It involves alternating short periods of intense or explosive anaerobic exercises separated by recovery periods (11). Despite confirming the positive effect of exercise training in preventing the onset of type 2 diabetes (T2D) and MetS, the molecular mechanisms involved in this field are completely unknown (7). It is known that miRNAs (microribonucleic acid) are involved in intercellular interactions; therefore, changes in the expression levels of miRNAs may reflect the details of changes in response to exercise. Thus, there is an urgent need to evaluate the response and function of new intercellular mediators, such as circulating miRNAs (12).
miRNAs (19-23 nucleotides) are single-stranded noncoding RNA molecules that play a role in the post-transcriptional control of the gene expression. miRNAs act as regulators of mRNA degradation and/or blocking protein translation through binding to their corresponding sequences within the 3’-untranslated regions (3'UTRs) (13). It has been manifested that miRNA, particularly serum miRNAs, are stable in blood circulation since they are resistant to RNase digestion. This shows great promise in becoming a novel diagnostic biomarker of MetS and its components (4, 6, 12, 14, 15). In the meantime, it has been shown that miR-21 and miR-122 are involved in glucose and lipid profile metabolism, liver disorders, and related physiological processes; thus, it has been suggested that interventions used to modulate these miRNAs can be a promising approach for treating metabolic diseases (16, 17). Kalaki-Jouybari et al. showed that HIIT is the best training method to improve the condition of NAFLD by inducing miR-122 compared to continuous training (18). After chronic endurance training, a decrease in the level of miR-21 in human plasma was observed (19). Also, the favorable effects of HIIT on alanine aminotransferase (ALT), aspartate aminotransferase (AST), lipid profile, and glucose metabolism have been shown (20, 21). However, it is still not clear whether HIIT can improve liver enzymes, glucose metabolic status, and lipid profile in patients with MetS by affecting the miR-122 and miR-21. Therefore, the effect of physical training on miR-122 and miR-21 in patients with MetS is unclear. Thus, this study aimed to explore the effect of HIIT training on changes in miR-21, miR-122, liver factors, lipid, and glucose factors in middle-aged men with MetS.
Methods
Participants
The current study was conducted in the city of Tabriz, East Azarbaijan, in northwest Iran. Before the training, each participant underwent a medical examination, which revealed no medical objections to physical exercise. This study was a quasi-experimental one with a pre-test/post-test design. The Statstodo web software was used to determine the sample size. Subjects (n=24) with MetS participated in this research. They met several criteria for being included (such as age range 40-50 years, body fat (BF%) 32%, having 3-5 metabolic risk indicators (waist circumference ≥ 102 cm, blood triglyceride (TG) ≥ 150 mg/dL, blood high-density lipoprotein (HDL) < 40 mg/dL, blood pressure ≥ 130/85 mmHg, and fasting blood glucose ≥ 110 mg/dL), BMI (body mass index) > 30, non-smoker, non-alcoholic, no chronic disease such as skeletal or coronary, and having received 2 doses of the COVID-19 vaccine). According to the BMI, maximal oxygen consumption) V̇O2max), and BF%, the subjects were randomly and homogeneously divided into 2 groups: control (n=12) and training (n=12). The BMI, maximal oxygen consumption) V̇O2max) and BF% were measured by the BMI formula, Brus test, and bio-impedance body composition analyzer (made in Korea, 721), respectively. During the research process, 3 subjects were excluded due to the lack of physical fitness required for the exercise protocol. The blood samples of 2 subjects were also removed due to a laboratory error. Finally, 9 people from the control group and 10 people from the training group completed the research process.
Volunteers received comprehensive information about the study protocol and procedures and provided written consent. The Ethics Committee of Sport Sciences Research Institute (SSRI) approved the study (REC-2110-1322 RI).
Training program
The beneficial effects of the exercise protocol used in the current research have already been confirmed on the factors affecting endothelial function and antioxidant capacity; hence, its effect on people with metabolic syndrome was investigated in the current study. The HIIT program was designed for 8 weeks, 3 sessions per week during the first 4 weeks and 4 sessions per week during the second 4 weeks, with an intensity of 80-90% heart rate (HR), and the exercises were done in a circle (22). In each training session, the exercises were performed in 5 sets, and the total training time was less than 60 minutes. The training program consisted of long knee, butterfly, burpee, kettlebell, and goblet squat exercises (Table 1).
Sampling and analysis methods
Blood samples were taken from the antecubital vein of the fasting subjects 48 hours before and after the last training session. Then, 10 mL of blood was collected, and serum factor profiles were assessed 48 hours before the intervention (after overnight fasting) and 48 hours after the last training session into EDTA (ethylenediaminetetraacetic acid)-containing tubes. Pars Azmoon kits and the photometric method by an autoanalyzer instrument (Abbott, model Alcyon 300, USA) were used for glucose (mg/dL), ALT (U/L), AST (U/L), TG (mg/dL), HDL-C (mg/dL), LDL-C (mg/dL), and cholesterol (mg/dL) measurement. After RNA extraction, miR-21 and miR-122 were measured according to the instructions of the Randox kit (nx 2332randox kit) and using Miller's method. Real-time polymerase chain reaction (qPCR) was performed to evaluate the miRNAs. A NanoDrop (ONEC model, made by the thermo American company) was used to evaluate the quantity and quality of RNAs, and the expression level of miRNAs was measured relative to the U6 reference gene. Finally, relative changes in gene expression levels were determined using the 2−ΔΔCT.
Statistical analysis
After collecting the data from 19 blood samples, analysis was done by using SPSS v. 25 (IBM Corp., Armonk, NY, USA) and Microsoft Office Excel v. 2016 (v16.0, USA). Data were presented as means ± standard deviations. Initially, the Shapiro-Wilk test was used to ensure the normal distribution of all the data. Moreover, a paired t-test was used to evaluate within-group changes (pre- and post-test data for each group), and the analysis of covariance (to remove interfering factors) was used to evaluate between-group changes (comparing the difference data between pre- and post-test of the groups) P<0.05.
Pearson's correlation was also used to evaluate the relationship between miRNAs and other factors (Tables 4 and 5).
Results
Discussion
In the present study, it was shown that after 8 weeks of HIIT training, the amount of miR-21 increased in men with MetS. So far, the studies conducted have reported different results, including an increase (23), a decrease (24), and no change (25). Alizadeh et al. reported an increase in miR-21 expression after 12 weeks of high-intensity aerobic interval exercise in women with breast cancer, which is in line with the results of the present study (23). The reason for contradictory results is the differences in subjects and types of training protocols. Regular physical activity usually shows a decrease in the expression of miR-21 in healthy people. However, in abnormal physiological conditions, due to the pathological change of miR-21 level in the baseline state, the results of the effect of exercise training can be contradictory. Researchers have shown that miR-21 is one of the most essential mammalian microRNAs. Besides, the expression level of miR-21 in liver and blood predicts the level of liver ischemia/reperfusion damage (26). Decreased steatosis, inflammation, and hepatic lipogenesis have been shown in miR-21 knockout mice (27). Nevertheless, in conditions of insulin resistance, miR-21 overexpression improved glucose metabolism and insulinemia through the PTEN/PI3K/Akt pathway (28) and increased insulin-induced glucose uptake in insulin-resistant adipocytes by promoting the translocation of the Glut4 plasma membrane (12, 14, 29). Also, it has been shown that in subjects with MetS, circulating levels of miR-21 were decreased compared to patients without MetS (30); therefore, the increasing effect of exercise training on miR-21 expression in people with MetS seems acceptable in the direction of modulating metabolic factors. Interestingly, in vivo treatment with miR-21 blocked high-fat-diet-induced weight gain in obese mice without altering food intake or physical activity. In addition, miR-21 significantly increases the expression of genes involved in adipose tissue browning and thermogenesis in 3T3-L1 adipocytes (31). The influence of miR-21 on the metabolic status, independently of the blood glucose condition, and through the regulation of several genes and biological processes, such as angiogenesis, vascular endothelial growth factor (VEGF) signaling, thermogenesis, browning, apoptosis, and adipogenesis, is involved in Adipose Tissue (AT) function (31).
The MiR-122 coding sequence comprises a single locus on the human chromosome 18 and is a liver-specific microRNA (32). MiR-122 is a key regulator of liver physiology by playing a central role in liver development, differentiation, and homeostasis. MiR-122 is also crucial in hepatic cholesterol homeostasis and adipose tissue metabolism. MiR-122 antagonism decreases the expression of several genes involved in hepatic lipid metabolism and cholesterol biosynthesis, including acetyl-CoA carboxylase alpha (ACC1), acetyl-CoA carboxylase beta (ACC2), ATP-citrate lyase (ACLY), stearoyl-CoA desaturase1 (SCD1), sterol regulatory element-binding protein 2 (SREBP2), FA synthase (FASN), and phosphomevalonate kin (PMVK). 1-Acylglycerol-3-phosphate O-acyltransferase 1 (AGPAT1) that regulates the energy state and CIDEC that controls triglyceride storage and FA oxidation have also been identified as potential targets of miR -122 (33, 34). Additionally, circulating miR-122 levels were elevated in subjects with metabolic syndrome or type 2 diabetes and correlated with high levels of saturated and monounsaturated fatty acids, delineating miR-122 as a marker of hepatic lipid metabolism disorders, including metabolic syndrome and type 2 diabetes (12, 35). In the present study, HIIT training led to a decrease in the miR-122 expression, which can indicate the regulatory effect of HIIT training on metabolism. De Mendonca et al. showed a decrease in circulating miR-122 expression after 8 weeks of aerobic training in obese rats (7). These researchers observed that circulating miR-122 increased in obese mice, and its expression returned to baseline after aerobic training (7). The increase of miR-122 following 8 weeks of HIIT training was reported by Kalaki-Jouybari et al. These researchers confirmed the essential role of miR-122 in lipid homeostasis through the regulation of lipogenic genes in TG metabolism and cholesterol biosynthesis pathway (18).
Circulating levels of miR-122 are associated with hepatic miR-122 expression level, liver damage, and levels of liver enzymes, such as ALT and AST (5, 36). Moreover, in people with metabolic disorders, the level of liver enzymes and metabolic risk factors, such as triglycerides, LDL, and cholesterol, are related to each other (37). Therefore, HIIT training can lead to the regulation of liver enzymes and metabolic risk factors by modulating and normalizing the expression of miR-21 and miR-122.
One of the possible mechanisms of the effects of HIIT training is exercise-induced metabolites, such as acetyl-CoA (for histone acetylation) and S-adenosyl-L-methionine (for methylation), which are essential for epigenetic modifications of white adipose tissue. The lactate produced during these exercises can change DNA methylation and miRNA expression, both in muscle tissue and in other tissues such as adipose tissue and liver. In this way, a part of miRNA changes and its effect on changes in blood lipid profiles can be justified (38, 39). In the present study, the changes in miRNAs after 8 weeks of HIIT indicate that exercise training exerts long-term adaptations on the profiles of circulating miRNAs. In this way, through stimulating the release of growth hormone, exercise training stimulates the synthesis of IGF-1 directly in muscles and indirectly in most tissues. While aging is associated with a decrease in levels and signaling of IGF1, it is possible that regular exercise training thereby causes changes in plasma miRNAs and inhibits age-related anabolic resistance. In fact, due to the nature of HIIT, these exercises can improve factors, such as insulin sensitivity, by affecting IGF1 signaling, changing the circulating miRNAs, reducing fat percentage values, increasing fat-free mass, and ultimately preventing the occurrence of age-related MetS (40). However, to confirm the results of the present study, more research is needed to confirm the effect of various types of exercise training on different ages and sexes.
Limitations
In this research, there were limitations, such as not controlling the subjects' motivation and their sleeping habits. In addition to controlling the above factors, future studies should apply caloric restriction as a second intervention along with exercise because caloric restriction can also have special effects on metabolic syndrome.
Conclusion
In this study, after 8 weeks of HIIT training, miR-122, glucose, TG, LDL, cholesterol, ALT, and AST were decreased, and miR-21 and HDL levels were increased. In addition, ideal changes in weight, fat percentage, BMI, and V̇O2max of the training group compared with the control group indicated the proper impact of the training on the individuals. In this study, the changes in lipid profiles and hepatic enzymes were in line with miR-122 changes. It was shown that miR-122 had a compensatory role before the 8 weeks of HIIT training protocol in subjects. Due to the constructive role of training in FA metabolism, miR-122 decreases and returns to the average level. Therefore, HIIT can improve the metabolic status under conditions associated with metabolic disorders through changes in miRNA levels.
Although only a few studies have been published in this field, among other factors, different methodologies in the experimental design, the exercise and training models, or the characteristics of the subjects (age, activity, history, etc.) have resulted in different outcomes, indicating that more research is required. Therefore, it is recommended that changes in the desired factors due to various exercise training and nutritional interventions be examined in different conditions
Acknowledgement
We thank and appreciate the cooperation of all the subjects for the implementation of the research training protocol.
Funding sources
None
Ethical statement
The Ethics Committee of Sport Sciences Research Institute (SSRI) approved the study (REC-2110-1322 RI). IRCT Id: IRCT20220128053844N1
Conflicts of interest
None.
Author contributions
Conceptualization: Hamid Reza Zolfi, Amir Shakib. Methodology: Hamid Reza Zolfi, Amir Shakib. Data collection: Amir Shakib, Zahra Niknam, Zhaleh Pashaei. Data analysis and interpretation: Amir Shakib, Zahra Niknam. Drafting the manuscript: Zahra Niknam, Amir Shakib, Zhaleh Pashaei, Hamid Reza Zolfi. All the authors have reviewed and approved the final version of the manuscript for submission.
The beneficial effects of the exercise protocol used in the current research have already been confirmed on the factors affecting endothelial function and antioxidant capacity; hence, its effect on people with metabolic syndrome was investigated in the current study. The HIIT program was designed for 8 weeks, 3 sessions per week during the first 4 weeks and 4 sessions per week during the second 4 weeks, with an intensity of 80-90% heart rate (HR), and the exercises were done in a circle (22). In each training session, the exercises were performed in 5 sets, and the total training time was less than 60 minutes. The training program consisted of long knee, butterfly, burpee, kettlebell, and goblet squat exercises (Table 1).
Blood samples were taken from the antecubital vein of the fasting subjects 48 hours before and after the last training session. Then, 10 mL of blood was collected, and serum factor profiles were assessed 48 hours before the intervention (after overnight fasting) and 48 hours after the last training session into EDTA (ethylenediaminetetraacetic acid)-containing tubes. Pars Azmoon kits and the photometric method by an autoanalyzer instrument (Abbott, model Alcyon 300, USA) were used for glucose (mg/dL), ALT (U/L), AST (U/L), TG (mg/dL), HDL-C (mg/dL), LDL-C (mg/dL), and cholesterol (mg/dL) measurement. After RNA extraction, miR-21 and miR-122 were measured according to the instructions of the Randox kit (nx 2332randox kit) and using Miller's method. Real-time polymerase chain reaction (qPCR) was performed to evaluate the miRNAs. A NanoDrop (ONEC model, made by the thermo American company) was used to evaluate the quantity and quality of RNAs, and the expression level of miRNAs was measured relative to the U6 reference gene. Finally, relative changes in gene expression levels were determined using the 2−ΔΔCT.
Statistical analysis
After collecting the data from 19 blood samples, analysis was done by using SPSS v. 25 (IBM Corp., Armonk, NY, USA) and Microsoft Office Excel v. 2016 (v16.0, USA). Data were presented as means ± standard deviations. Initially, the Shapiro-Wilk test was used to ensure the normal distribution of all the data. Moreover, a paired t-test was used to evaluate within-group changes (pre- and post-test data for each group), and the analysis of covariance (to remove interfering factors) was used to evaluate between-group changes (comparing the difference data between pre- and post-test of the groups) P<0.05.
Pearson's correlation was also used to evaluate the relationship between miRNAs and other factors (Tables 4 and 5).
Results
The individual and anthropometric characteristics of the subjects are descriptively shown in Table 1. The blood index values measured before and after 8 weeks of HIIT, respectively, are given in Table 2.
Table 2. The comparison of anthropometric and physiological characteristics of the control (n=9) and training groups (n=10) before and after the 8-week high-intensity interval training |
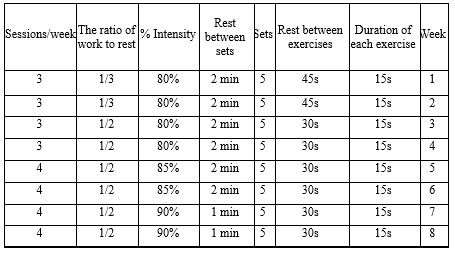
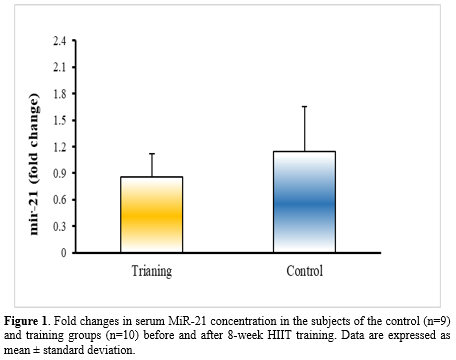
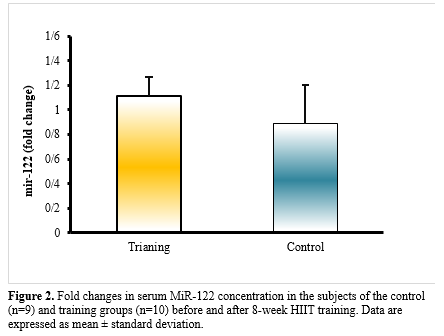
According to the paired t-test and analysis of covariance, weight (P<0.001), BMI (P<0.001), fat% (P<0.001), V̇O2max (P=0.026), serum levels of glucose (P<0.001), triglyceride (P=0.002), cholesterol (P=0.001), LDL (P<0.001), HDL (P˂0.001), ALT (P=0.001), AST (P=0.002), miR-21 (P<0.001), and miR-122 (P˂0.001) for the subjects differed significantly after 8 weeks of HIIT training compared to the pre-test (Tables 2 and 3, Figures 1 and Figure 2). Also, all the data showed a significant difference between the control and training groups (P˂0.05) (Tables 2 and 3). Significant differences (P˂0.05) between groups for the post-test data of miR-21 and miR-122 are shown in Figures 1 and 2.
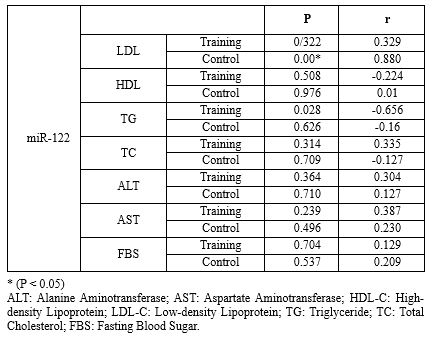
There was no significant relationship among the studied factors, while the relationship between miR-122 and triglyceride was significant (P=0.028) (Tables 4 and 5).

Table 3. The comparison of mean blood indices measured in the subjects of control (n=9) and training groups (n=10) before and after the 8-week high-intensity interval training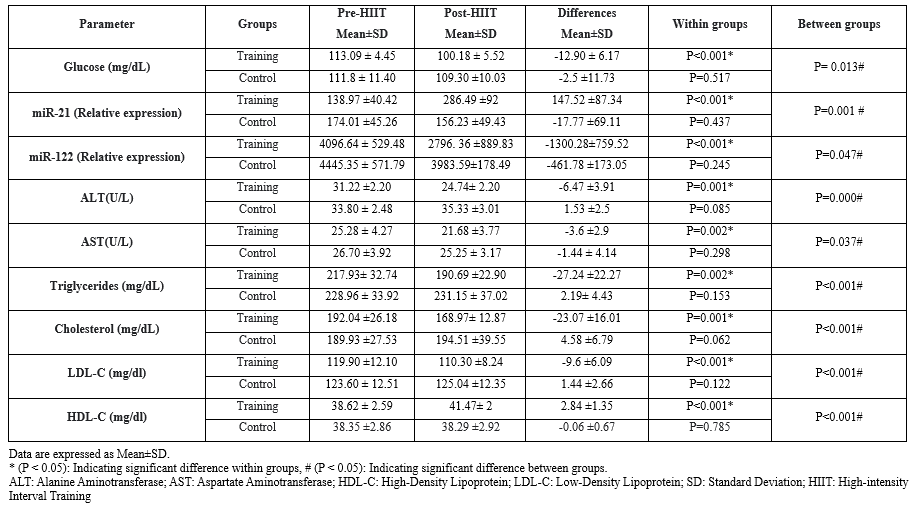 |
Table 4. Pearson correlation between MiR-21 with other factors in the subjects of control (n=9) and training groups (n=10) before and after the 8-week high-intensity interval training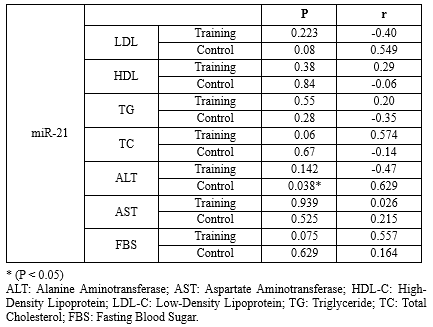 |
|
Table 5. Pearson correlation between MiR-122 with other factors in the subjects of control (n=9) and training groups (n=10) before and after the 8-week HIIT training.
 |
Discussion
In the present study, it was shown that after 8 weeks of HIIT training, the amount of miR-21 increased in men with MetS. So far, the studies conducted have reported different results, including an increase (23), a decrease (24), and no change (25). Alizadeh et al. reported an increase in miR-21 expression after 12 weeks of high-intensity aerobic interval exercise in women with breast cancer, which is in line with the results of the present study (23). The reason for contradictory results is the differences in subjects and types of training protocols. Regular physical activity usually shows a decrease in the expression of miR-21 in healthy people. However, in abnormal physiological conditions, due to the pathological change of miR-21 level in the baseline state, the results of the effect of exercise training can be contradictory. Researchers have shown that miR-21 is one of the most essential mammalian microRNAs. Besides, the expression level of miR-21 in liver and blood predicts the level of liver ischemia/reperfusion damage (26). Decreased steatosis, inflammation, and hepatic lipogenesis have been shown in miR-21 knockout mice (27). Nevertheless, in conditions of insulin resistance, miR-21 overexpression improved glucose metabolism and insulinemia through the PTEN/PI3K/Akt pathway (28) and increased insulin-induced glucose uptake in insulin-resistant adipocytes by promoting the translocation of the Glut4 plasma membrane (12, 14, 29). Also, it has been shown that in subjects with MetS, circulating levels of miR-21 were decreased compared to patients without MetS (30); therefore, the increasing effect of exercise training on miR-21 expression in people with MetS seems acceptable in the direction of modulating metabolic factors. Interestingly, in vivo treatment with miR-21 blocked high-fat-diet-induced weight gain in obese mice without altering food intake or physical activity. In addition, miR-21 significantly increases the expression of genes involved in adipose tissue browning and thermogenesis in 3T3-L1 adipocytes (31). The influence of miR-21 on the metabolic status, independently of the blood glucose condition, and through the regulation of several genes and biological processes, such as angiogenesis, vascular endothelial growth factor (VEGF) signaling, thermogenesis, browning, apoptosis, and adipogenesis, is involved in Adipose Tissue (AT) function (31).
The MiR-122 coding sequence comprises a single locus on the human chromosome 18 and is a liver-specific microRNA (32). MiR-122 is a key regulator of liver physiology by playing a central role in liver development, differentiation, and homeostasis. MiR-122 is also crucial in hepatic cholesterol homeostasis and adipose tissue metabolism. MiR-122 antagonism decreases the expression of several genes involved in hepatic lipid metabolism and cholesterol biosynthesis, including acetyl-CoA carboxylase alpha (ACC1), acetyl-CoA carboxylase beta (ACC2), ATP-citrate lyase (ACLY), stearoyl-CoA desaturase1 (SCD1), sterol regulatory element-binding protein 2 (SREBP2), FA synthase (FASN), and phosphomevalonate kin (PMVK). 1-Acylglycerol-3-phosphate O-acyltransferase 1 (AGPAT1) that regulates the energy state and CIDEC that controls triglyceride storage and FA oxidation have also been identified as potential targets of miR -122 (33, 34). Additionally, circulating miR-122 levels were elevated in subjects with metabolic syndrome or type 2 diabetes and correlated with high levels of saturated and monounsaturated fatty acids, delineating miR-122 as a marker of hepatic lipid metabolism disorders, including metabolic syndrome and type 2 diabetes (12, 35). In the present study, HIIT training led to a decrease in the miR-122 expression, which can indicate the regulatory effect of HIIT training on metabolism. De Mendonca et al. showed a decrease in circulating miR-122 expression after 8 weeks of aerobic training in obese rats (7). These researchers observed that circulating miR-122 increased in obese mice, and its expression returned to baseline after aerobic training (7). The increase of miR-122 following 8 weeks of HIIT training was reported by Kalaki-Jouybari et al. These researchers confirmed the essential role of miR-122 in lipid homeostasis through the regulation of lipogenic genes in TG metabolism and cholesterol biosynthesis pathway (18).
Circulating levels of miR-122 are associated with hepatic miR-122 expression level, liver damage, and levels of liver enzymes, such as ALT and AST (5, 36). Moreover, in people with metabolic disorders, the level of liver enzymes and metabolic risk factors, such as triglycerides, LDL, and cholesterol, are related to each other (37). Therefore, HIIT training can lead to the regulation of liver enzymes and metabolic risk factors by modulating and normalizing the expression of miR-21 and miR-122.
One of the possible mechanisms of the effects of HIIT training is exercise-induced metabolites, such as acetyl-CoA (for histone acetylation) and S-adenosyl-L-methionine (for methylation), which are essential for epigenetic modifications of white adipose tissue. The lactate produced during these exercises can change DNA methylation and miRNA expression, both in muscle tissue and in other tissues such as adipose tissue and liver. In this way, a part of miRNA changes and its effect on changes in blood lipid profiles can be justified (38, 39). In the present study, the changes in miRNAs after 8 weeks of HIIT indicate that exercise training exerts long-term adaptations on the profiles of circulating miRNAs. In this way, through stimulating the release of growth hormone, exercise training stimulates the synthesis of IGF-1 directly in muscles and indirectly in most tissues. While aging is associated with a decrease in levels and signaling of IGF1, it is possible that regular exercise training thereby causes changes in plasma miRNAs and inhibits age-related anabolic resistance. In fact, due to the nature of HIIT, these exercises can improve factors, such as insulin sensitivity, by affecting IGF1 signaling, changing the circulating miRNAs, reducing fat percentage values, increasing fat-free mass, and ultimately preventing the occurrence of age-related MetS (40). However, to confirm the results of the present study, more research is needed to confirm the effect of various types of exercise training on different ages and sexes.
Limitations
In this research, there were limitations, such as not controlling the subjects' motivation and their sleeping habits. In addition to controlling the above factors, future studies should apply caloric restriction as a second intervention along with exercise because caloric restriction can also have special effects on metabolic syndrome.
Conclusion
In this study, after 8 weeks of HIIT training, miR-122, glucose, TG, LDL, cholesterol, ALT, and AST were decreased, and miR-21 and HDL levels were increased. In addition, ideal changes in weight, fat percentage, BMI, and V̇O2max of the training group compared with the control group indicated the proper impact of the training on the individuals. In this study, the changes in lipid profiles and hepatic enzymes were in line with miR-122 changes. It was shown that miR-122 had a compensatory role before the 8 weeks of HIIT training protocol in subjects. Due to the constructive role of training in FA metabolism, miR-122 decreases and returns to the average level. Therefore, HIIT can improve the metabolic status under conditions associated with metabolic disorders through changes in miRNA levels.
Although only a few studies have been published in this field, among other factors, different methodologies in the experimental design, the exercise and training models, or the characteristics of the subjects (age, activity, history, etc.) have resulted in different outcomes, indicating that more research is required. Therefore, it is recommended that changes in the desired factors due to various exercise training and nutritional interventions be examined in different conditions
Acknowledgement
We thank and appreciate the cooperation of all the subjects for the implementation of the research training protocol.
Funding sources
None
Ethical statement
The Ethics Committee of Sport Sciences Research Institute (SSRI) approved the study (REC-2110-1322 RI). IRCT Id: IRCT20220128053844N1
Conflicts of interest
None.
Author contributions
Conceptualization: Hamid Reza Zolfi, Amir Shakib. Methodology: Hamid Reza Zolfi, Amir Shakib. Data collection: Amir Shakib, Zahra Niknam, Zhaleh Pashaei. Data analysis and interpretation: Amir Shakib, Zahra Niknam. Drafting the manuscript: Zahra Niknam, Amir Shakib, Zhaleh Pashaei, Hamid Reza Zolfi. All the authors have reviewed and approved the final version of the manuscript for submission.
Editorial: Original article |
Subject:
Health
Received: 2023/07/9 | Accepted: 2023/11/27 | Published: 2023/12/22
Received: 2023/07/9 | Accepted: 2023/11/27 | Published: 2023/12/22
References
1. Bovolini A, Garcia J, Andrade MA, Duarte JA. Metabolic syndrome pathophysiology and predisposing factors. Int J Sports Med. 2021;42(3):199-214. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Barahimi H, Esmaeilzadeh A, Rajaei F, Hasanzadeh A, Kafeshani O. Association of dietary pattern and metabolic syndrome in 15-to 49-years-old women. Journal of Isfahan Medical School. 2015;33(322):70-81. [View at Publisher] [Google Scholar]
3. Ostovar R, Kiani F, Sayehmiri F, Yasemi M, Mohsenzadeh Y, Mohsenzadeh Y. Prevalence of metabolic syndrome in Iran: A meta-analysis. Electron physician. 2017;9(10):5402-18. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Huang Y, Yan Y, Xv W, Qian G, Li C, Zou H, et al. A new insight into the roles of MiRNAs in metabolic syndrome. Biomed Res Int. 2018;2018:7372636. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Suehiro T, Miyaaki H, Kanda Y, Shibata H, Honda T, Ozawa E, et al. Serum exosomal microRNA‑122 and microRNA‑21 as predictive biomarkers in transarterial chemoembolization‑treated hepatocellular carcinoma patients. Oncol Lett. 2018;16(3):3267-73. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Lin H, Mercer KE, Ou X, Mansfield K, Buchmann R, Børsheim E, et al. Circulating microRNAs are associated with metabolic markers in adolescents with hepatosteatosis. Front Endocrinol. 2022;13:856973. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. de Mendonça M, Rocha KC, de Sousa É, Pereira BM, Oyama LM, Rodrigues AC. Aerobic exercise training regulates serum extracellular vesicle miRNAs linked to obesity to promote their beneficial effects in mice. Am J Physiol Endocrinol Metab. 2020;319(3):E579-91. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Silva GJ, Bye A, El Azzouzi H, Wisløff U. MicroRNAs as important regulators of exercise adaptation. Prog Cardiovasc Dis. 2017;60(1):130-51. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Xie WQ, Men C, He M, Li Ys, Lv S. The Effect of MicroRNA-Mediated Exercise on Delaying Sarcopenia in Elderly Individuals. Dose Response. 2020;18(4):1559325820974543. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Bahram ME, Afroundeh R, Ghiyami Taklimi SH, Sadeghi A, Gholamhosseini M. Effect of High-intensity Interval Training and Loquat Leaf Extract Consumption on Liver Enzymes in Obese Men With Non-alcoholic Fatty Liver Disease. Complement Med J. 2021;11(2):102-15. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Rhibi F, Abderrahman AB, Prioux J, Clark CCT, Bideau B, Besbes S, et al. Effects of different training intensities in high-intensity interval training (HIIT) on maximal aerobic velocity, hematological and muscle-damage markers in healthy young adults. BMC Sports Sci Med Rehabil. 2022;14(1):158. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Fernández-Sanjurjo M, de Gonzalo-Calvo D, Díez-Robles S, Dávalos A, Iglesias-Gutiérrez E. Circulating microRNA as regulators of the molecular response in exercise in healthy people. Medicina Del Deporte. 2016;33(6):N176. [View at Publisher] [Google Scholar]
13. Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451-65. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Jenike AE, Halushka MK. miR-21: a non‐specific biomarker of all maladies. Biomark Res. 2021;9(1):18. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Fang Y, Yan D, Wang L, Zhang J, He Q. Circulating microRNAs (miR‐16, miR‐22, miR‐122) expression and early diagnosis of hepatocellular carcinoma. J Clin Lab Anal. 2022;36(7):e24541. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Rotllan N, Price N, Pati P, Goedeke L, Fernández-Hernando C. microRNAs in lipoprotein metabolism and cardiometabolic disorders. Atherosclerosis. 2016;246:352-60. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Yamada H, Suzuki K, Ichino N, Ando Y, Sawada A, Osakabe K, et al. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin Chim Acta. 2013;424:99-103. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Kalaki-Jouybari F, Shanaki M, Delfan M, Gorgani-Firouzjae S, Khakdan S. High-intensity interval training (HIIT) alleviated NAFLD feature via miR-122 induction in liver of high-fat high-fructose diet induced diabetic rats. Arch Physiol Biochem. 2020;126(3):242-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Nielsen S, Åkerström T, Rinnov A, Yfanti C, Scheele C, Pedersen BK, et al. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PloS One. 2014;9(2):e87308. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. LU-Xin X-B. Effect of functional high-intensity interval training combined with inhalation dietary intervention on obese college students. Chinese Journal of School Health. 2021;(12):569-73. [View at Publisher] [DOI] [Google Scholar]
21. Pashaei J, Jafari12 A, Alivand MR. Effect of tow types of high-intensity interval training with and without resistance training on lipid profiles and glucose homeostasis in overweight/obese middle-aged women. Research in Medicine: Journal of Research in Medical Sciences. 2020;44(4). [View at Publisher] [Google Scholar]
22. Zolfi H, Shakib A. The effect of the high intensity interval training on endothelial function concentrating on alterations in miR-16 expression, total antioxidant capacity and serum malondialdehyde in the obese men. Feyz Med Sci J. 2022;26(4):435-45. [View at Publisher] [DOI] [Google Scholar]
23. Alizadeh S, Isanejad A, Sadighi S, Khalighfard S, Alizadeh AM. Effect of a high-intensity interval training on serum microRNA levels in women with breast cancer undergoing hormone therapy. A single-blind randomized trial. Ann Phys Rehabil Med. 2019;62(5):329-35. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Horak M, Zlamal F, Iliev R, Kucera J, Cacek J, Svobodova L, et al. Exercise-induced circulating microRNA changes in athletes in various training scenarios. PloS One. 2018;13(1):e0191060. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Li Y, Yao M, Zhou Q, Cheng Y, Che L, Xu J, et al. Dynamic regulation of circulating microRNAs during acute exercise and long-term exercise training in basketball athletes. Front Physiol. 2018;9:282. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Salah A, Karimi MH, Sajedianfard J, Nazifi S, Yaghobi R. Expression pattern of MicroRNA-21 during the liver ischemia/reperfusion. Iran J Allergy Asthma Immunol. 2021;20(1):88-97. [View at Publisher] [DOI] [PMID] [Google Scholar]
27. Albracht-Schulte K, Gonzalez S, Jackson A, Wilson S, Ramalingam L, Kalupahana NS, et al. Eicosapentaenoic acid improves hepatic metabolism and reduces inflammation independent of obesity in high-fat-fed mice and in HepG2 cells. Nutrients. 2019;11(3):599. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Ling HY, Hu B, Hu XB, Zhong J, Feng SD, Qin L, et al. MiRNA-21 reverses high glucose and high insulin induced insulin resistance in 3T3-L1 adipocytes through targeting phosphatase and tensin homologue. Exp Clin Endocrinol Diabetes. 2012;120(9):553-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Liu R, Liu C, He X, Sun P, Zhang B, Yang H, et al. MicroRNA-21 promotes pancreatic β cell function through modulating glucose uptake. Nat Commun. 2022;13(1):3545. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. He QF, Wang LX, Zhong JM, Hu RY, Fang L, Wang H, et al. Circulating microRNA-21 is downregulated in patients with metabolic syndrome. Biomed Environ Sci. 2016;29(5):385-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Lhamyani S, Gentile AM, Giráldez-Pérez RM, Feijóo-Cuaresma M, Romero-Zerbo SY, Clemente-Postigo M, et al. miR-21 mimic blocks obesity in mice: A novel therapeutic option. Mol Ther Nucleic Acids. 2021;26:401-16. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Jopling C. Liver-specific microRNA-122: Biogenesis and function. RNA Biol. 2012;9(2):137-42. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Fernández-Tussy P, Ruz-Maldonado I, Fernández-Hernando C. MicroRNAs and circular RNAs in lipoprotein metabolism. Curr Atheroscler Rep. 2021;23(7):33. [View at Publisher] [DOI] [PMID] [Google Scholar]
34. Hu Y, Du G, Li G, Peng X, Zhang Z, Zhai Y. The miR-122 inhibition alleviates lipid accumulation and inflammation in NAFLD cell model. Arch Physiol Biochem. 2021;127(5):385-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
35. Improta-Caria AC, Aras Júnior R. Physical Exercise Training and Chagas Disease: Potential Role of MicroRNAs. Arq Bras Cardiol. 2021;117(1):132-41. [View at Publisher] [DOI] [PMID] [Google Scholar]
36. Caster PV, Brandenburger T, Strahl T, Metzger S, Bauer I, Pannen B, et al. Circulating microRNA‑122,‑21 and‑223 as potential markers of liver injury following warm ischaemia and reperfusion in rats. Mol Med Rep. 2015;12(2):3146-50. [View at Publisher] [DOI] [PMID] [Google Scholar]
37. Omagari K, Kadokawa Y, Masuda JI, Egawa I, Sawa T, Hazama H, et al. Fatty liver in non‐alcoholic non‐overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002;17(10):1098-105. [View at Publisher] [DOI] [PMID] [Google Scholar]
38. Cruciani S, Delitala AP, Cossu ML, Ventura C, Maioli M. Management of Obesity and Obesity-Related Disorders: From Stem Cells and Epigenetics to Its Treatment. Int J Mol Sci. 2023;24(3):2310. [View at Publisher] [DOI] [PMID] [Google Scholar]
39. Barrón-Cabrera E, Ramos-Lopez O, González-Becerra K, Riezu-Boj JI, Milagro FI, Martínez-López E, et al. Epigenetic modifications as outcomes of exercise interventions related to specific metabolic alterations: a systematic review. Lifestyle Genom. 2019;12(1-6):25-44. [View at Publisher] [DOI] [PMID] [Google Scholar]
40. Nair VD, Ge Y, Li S, Pincas H, Jain N, Seenarine N, et al. Sedentary and trained older men have distinct circulating exosomal microRNA profiles at baseline and in response to acute exercise. Front Physiol. 2020;11:605. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |