Volume 11, Issue 1 (7-2023)
Jorjani Biomed J 2023, 11(1): 3-8 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
pirmoradi S. Ligand-based pharmacophore modeling to identify plant-derived acetylcholinesterase inhibitor natural compounds in Alzheimer’s disease. Jorjani Biomed J 2023; 11 (1) :3-8
URL: http://goums.ac.ir/jorjanijournal/article-1-952-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-952-en.html
Department of Biochemistry, Shahid Chamran University of Ahvaz, Ahvaz, Iran. , pirmoradi150@gmail.com
Full-Text [PDF 979 kb]
(1845 Downloads)
| Abstract (HTML) (4297 Views)
Full-Text: (859 Views)
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly and is characterized by loss of memory and other cognitive functions. It is a neurodegenerative disease characterized by decreased cognitive function in patients due to forming Aβ peptides and neurofibrillary tangles (NFT) in the brain (1). AD and other age-related memory disorders have always attracted the attention of researchers worldwide as a slowly progressive disorder characterized by the appearance of neurofibrillary tangles, neural plaques, rapid loss of synapses, degeneration of primary cholinergic neurons, and impaired reasoning. Planning, perception, and thinking are defined. Moreover, it is associated with some metabolic disorders (2,3). This disease becomes a big challenge for the health and social care system and a significant economic burden in the future (4). Therefore, the need to develop new treatments can reduce this risk. Acetylcholinesterase, known as AChE, is an essential enzyme in the family of serine hydrolases that plays a crucial role in memory and cognition (5,6). Cholinesterase is one of the targets used in the design of new drugs for the treatment of AD since the nerve cells that secrete this neurotransmitter substance are among the first cells affected by the pathological changes of AD and are destroyed because the cholinergic neurons located in the basal forebrain, including the neurons that form the basal nucleus form Meinert, which is severely lost in AD. Therefore, it seems that preventing the breakdown of acetylcholine by inhibiting the cholinesterase enzyme can be vital in stabilizing memory and thinking power (7). So far, only seven drugs, capractamine, donepezil, galantamine, huperzine, memantine, rivastigmine, and tacrine (8,9), have been approved by the Food and Drug Administration (FDA) for treating AD. Due to numerous side effects such as hepatotoxicity, gastrointestinal disorders, dizziness, diarrhea, vomiting, nausea, and pharmacokinetic disadvantages, there are many limitations in using these treatment options for AD, a significant reason for discovering new, more effective compounds (10). Growing evidence shows that natural compounds are useful for studying the inhibitory effect on AChE activity. Researchers are constantly trying to find new medicines that, in addition to having better effects, have fewer side effects, and plants have always been essential sources for finding new drugs with acetylcholine enzyme inhibitory effects, which have been of interest. Plants provide materials rich in bioactive compounds, which can be considered a basic strategy for treating various diseases, such as AD (11). Thus, during various studies, several plant chemicals, namely alkaloids, pregnane glycosides (cynancoids), stilbenes, triterpenes (12), ursane (13), and xanthones have shown AChE inhibitory activity. In general, many families of different plants, such as Asteraceae (14), Menispermaceae (15), Malvaceae (16), Zingibraceae (17), and Hypericaceae, have been subjected to various studies, and the extracts and secondary compounds obtained from their different parts have been able to get promising results, to inhibit the activity of the acetylcholinesterase enzyme and to provide researchers with the treatment of AD. The development of new drugs with many clinical applications is needed to reduce the consequences of many diseases. Today, scientists generate large sets of 3D structural data and several leaders and reference therapeutic compounds.

Hence, the drug discovery process requires virtual screening of drugs, molecular docking, and molecular dynamics simulation studies to deal with such a large data set and design new therapeutic compounds. Virtual screening combined with molecular and binding modeling helps to develop new enzyme inhibitors and pharmacophores that bind to receptor sites (18). In this study, natural molecules were collected and identified based on qualitative and quantitative pharmacophoric characteristics and AChE inhibition through databases containing natural compounds and the pharmite server. The analysis results and the best inhibitory compounds were identified for research and subjected to additional analysis and investigation.
Methods
Preparation of Acetylcholinesterase Complex with Donepezil enzyme files and ligand design
This study first prepared the PDB structure (three-dimensional structure) of the Acetylcholinesterase Complex with Donepezil using its access code from the RCSB database. Then, this complex was selected as the leader compound for the virtual pharmacophore search.
Preparation of receptor-ligand for the pharmacophore process
In this step, using the pharmit server, the complex containing the drug-inhibiting receptor was prepared for the pharmacophore process, and the receptor was also prepared through SPDBV.4.1 and MVD software.
Pharmacophore modeling
In this step, inhibitor/receptor files were called by ZINC Pharmer and Pharmit databases and used as a template to find new inhibitory ligands to inhibit the desired 7E3H enzyme. Then, all the inhibitory ligands were retrieved from these databases based on this main inhibitory template called donepezil, and the ligands that had a high similarity to the template ligand were selected for the docking process so that donepezil was confirmed. It searches databases containing natural compounds and finds natural ligands similar to donepezil’s model drug for docking.
Checking the stability of the receptor composition
At this stage, using Iuperd2 software, the sequence structure of the renin enzyme file and its stability level were investigated to ensure stable inhibition with inhibitory ligands (19).
Docking and visualization of ligand/receptor interactions
At this stage, using MVD and AutoDock vina software, the docking process between the receptor and a number of the best inhibitory ligands was selected from among all the selected ligands by the pharmacophore. Finally, ligand/receptor interactions were shown by Discovery Studio software.
Investigating the ADME characteristics of the reference compound and selected superior ligands
At this stage, using some software and servers such as molsoft, PKCSM swiss ADME, and ADMEtlab 2.0, molecular properties and drug similarity, toxicity, absorption, metabolism, secretion, and release of inhibitory ligand compounds were investigated.
Results
Results of preparation of Acetylcholinesterase Complex with Donepezil enzyme files and ligand design
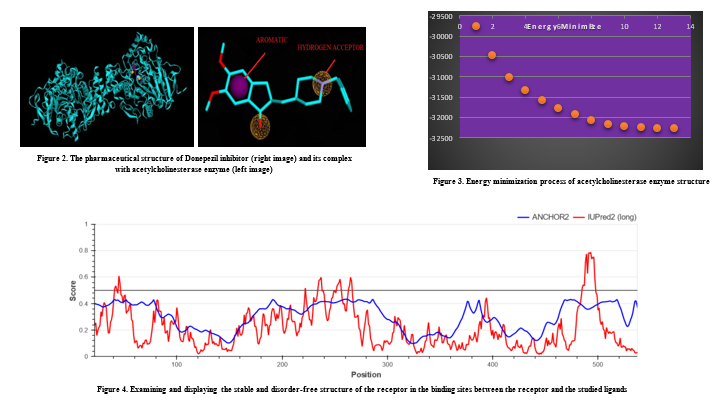
In this study, the PDB structure (three-dimensional structure) of the acetylcholinesterase enzyme compound with the access code (7E3H) was prepared from the RCSB database (20). Then, by studying various articles on the inhibitory compounds of acetylcholinesterase, the donepezil compound, the vital approved drug to inhibit this enzyme, was selected as the leader compound for the virtual pharmacophoric search (Figure 2).
The results of receptor-ligand preparation for the pharmacophore process
At this stage, using SPDV-4.1 and MVD software, the studied ligands and receptor were minimized in terms of energy, and the heteroatoms of the receptor were removed to perform the pharmacophore process. Furthermore, the leader ligand and its receptor, the enzyme acetylcholinesterase, were prepared (Figure 3).
Results of pharmacophore and virtual search
The acetylcholinesterase enzyme, along with its inhibitor Donepezil (7E3H), is listed in the Pharmit database. Essential features for creating pharmacophore models in the desired complex, such as aromatic rings, hydrophobic bonds, hydrogen bond donors, and hydrogen bond acceptors, were analyzed (21). Thus, through the pharmit server and the databases of natural compounds in it, based on the characteristics of the descriptors of the model complex, among more than 88,619 ligands in this server, finally, based on the characteristics and degree of similarity with the model ligand, Donepezil, from the database of natural compounds, six ligands were prepared and selected for further analysis for the molecular docking process, all of which followed Lipinski’s five rules and had good ADMET properties.
The results of investigating the stability of the receptor composition
The results of the IUPred2 software show a stable structure and no disorder in the receptor in the binding sites between the receptor and the studied ligands (Figure 4).
Docking results and display of ligand/receptor interactions
During the docking process, the complexes with the lowest energy were selected from among the results (Table 1).
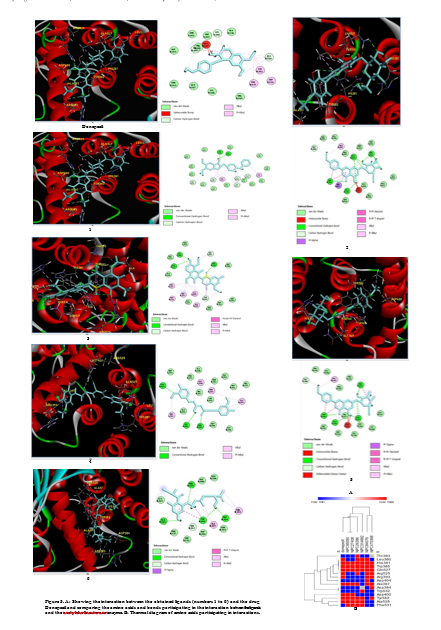
Then, with the help of different software such as Discovery Studio, all the interactions resulting from docking selected top ligands with acetylcholinesterase enzyme were illustrated. All the essential links participating in these interactions were displayed, and finally, the vital amino acids participating in all the ligands were shown and identified by drawing a thermal diagram (Figure 5).
The results of investigating the ADME characteristics of the reference compound and selected superior ligands
The following results were obtained using molsoft, PKCSM, swissADME, and ADMEtlab 2.0 servers, investigating the molecular properties, drug similarity, toxicity, absorption, metabolism, secretion, and release of inhibitory ligand compounds. According to the obtained results, the obtained ligands all had gastrointestinal absorption. In addition, ligands 1, 3, 4, and 6 had good solubility in fats compared to donepezil, and the obtained ligands followed Lipinski’s principles and had favorable solubility and acceptable pharmaceutical similarity (Table 2).


Discussion
Many efforts have been focused on finding new ligands based on natural compounds that can bind to the active site of enzymes to find stronger reversible inhibitors. Therefore, investigating and predicting the interactions between small molecules and enzyme proteins can be vital for deciphering many biological processes and plays an important role in discovering new drugs with fewer side effects. On the other hand, much evidence has shown that among the types of native and natural plants, the organic parts obtained from the extracts of some different medicinal plants have shown a significant inhibitory effect on AChE. Plants provide materials rich in bioactive compounds, which can be considered a basic strategy for treating various diseases, such as AD (22,23). However, nowadays, laboratory and experimental investigation of anti-enzyme properties of medicinal plants and their effective compounds is time-consuming, expensive, and with the possibility of human error. Therefore, in recent years, the introduction of complementary or alternative methods of experimental methods, such as bioinformatics and computational methods, has received much attention. This research used the pharmacophore process and virtual search in the format database, ZincPharmer. Besides, based on the pharmacophore and structural characteristics of the reference acetylcholinesterase inhibitor compound (donepezil), a large number of natural compound ligands were obtained through virtual search. During the molecular binding process between these ligand compounds, the obtained ligand was done with acetylcholinesterase enzyme, and the best binding energy between the ligands and the receptor was selected from among them (24). Among thousands of natural compounds, finally, the top six ligands based on the reference compound of acetylcholinesterase inhibitor (donepezil) were subsequently selected as a suitable binding model with AChE by using molecular docking methods and through various bioinformatics tools and based on scores. Finally, their interaction with the active site of the AChE enzyme was displayed using the Discovery Studio program (25). In general, many studies have shown therapeutic effects on AD. One study found that a group of plants that have been reported to have the biological effects of the abundance of plants rich in terpene and coumarin compounds are the plants of the genus Ferula. Another study found that the essential oil of Thymus vulgaris or garden thyme, a plant from the mint family, contains phenols such as thymol, carvacrol, simene, linalool, and pinene, whose anti-Alzheimer effect has been investigated, and proven. In other studies, it has been determined that lemon balm, with the scientific name L. Melissa officinalis, is a plant from the mint family used in traditional Iranian medicine in treating a wide range of diseases, including neurological diseases such as amnesia, epilepsy, paralysis, stroke, migraine, and vertigo. Many pharmacological studies show the neuroprotective effects of lemon balm and its main active ingredient (rosmarinic acid). Lemon balm exerts its effects with different mechanisms, including suppressing oxidative stress, inhibiting acetylcholinesterase, stimulating acetylcholine and GABA-A receptors, and inhibiting metalloproteinase-2 and monoamine oxidase enzymes (26). During the results of the docking process, it was shown that ligand 4, which had a higher binding energy than the others (VINA SCORE=-11) and with the acetylcholinesterase enzyme through normal hydrogen interactions with amino acids ARG525, GLN527, ASP404, and also through p-alkyl and alkyl bonds. Through HIS381, ALA397, LEU380, and ARG393, it has established important interactions with the acetylcholinesterase enzyme, most of which established bonds similar to the reference compound with the acetylcholinesterase enzyme (Figures 4, 5) and had a high interaction similarity with the reference ligand. The other top ligand was ligand 2 (VINA SCORE=-10), showing the most structural and interaction similarity with the reference inhibitory ligand. This ligand has three hydrogen bonds, LEU380, GLN527, and ARG525, and two hydrogen/carbon bonds, TYR382. In addition, HIS381 had pi-alkyl interactions,
and PHE531, HIS381, ALA528, ALA397, and a pi-Omid bond through TYR382 (Figures 2-5). Another significant ligand with high similarity to the reference compound in terms of interactions was ligand number 5 (VINASCORE=-10.5). This ligand has four hydrogen bonds, ALA528, TYR382, GLN527, HIS381, and a hydrogen/carbon bond ASP400. In addition, it has pi-alkyl interaction with ALA528 and ALA397, and It has established a sigma-pi bond through TYR382 and a pi-pi-shaped, pi-pi-stacked bond with the amino acid TYR382 (Figure 5).In general, among the six selected ligands, these two ligands, numbers 2 and 5, had the most interaction similarity with the reference ligand, and they probably show a good inhibitory effect, specifically ligand 5 (Figure 4B). Ligand number 2 is obtained from the Asteraceae family. Besides, in a study, it was found that the secondary compounds obtained from different parts of this family of plants, such as the flavonoid compounds found in Silybum marianum, show the potential to inhibit the accumulation of AChE and Aβ peptides (27), revealed by a series of assays, including AChE inhibition assays, as well as in vivo and silico docking studies. Mice treated with silibinin performed better than control mice in various tests. In addition, molecular dynamics simulations indicated that silybinin has a dual inhibitory function against AChE and Aβ peptide accumulation (28). In another study investigating the effect of the methanolic extract of another plant from this family called Phagnalon saxatile, the inhibitory power of various compounds of this extract was shown to inhibit both AChE and BChE enzymes. During the purification of the methanolic extract of this plant, it was found that this extract contains several phenolic compounds, among which luteolin and 3,5-caffeoylquinic acid showed the strongest inhibitory activity against AChE and caffeic acid and luteolin against BChE (29). Furthermore, in another study, chloroform and methanol extracts of Pulicaria stephanocarpa from this family of plants were tested for AChE inhibition, and the results showed that chloroform extract revealed more inhibitory activity (30). Another study tested the ethanolic extract of Artemisia annua, four fractions, and five isolated compounds for AChE inhibitory activity. Two of the four fractions were effective as AChEI inhibitors. However, two of the five isolated compounds - artemisinin (31) and chrysosplantin (32) were the most potent and had the highest AChE inhibitory activity (33). Ligand No. 5 is a plant from the Malvaceae family, and various studies report the effectiveness of different species’ extracts in inhibiting the acetylcholinesterase enzyme. It was observed that the extract showed the effects of inhibiting AChE (34). In another study, the whole plant of Sida rhombifolia Linn was extracted with methanol, ethyl acetate, and n-hexane, and it was found that the n-hexane extract had the highest inhibitory effect on AChE with an inhibition percentage of 68.5% (35). Docking results show that each of these compounds interacts with residues involved in substrate selectivity and catalytic activity, affecting the physicochemical properties and spatial orientation in the binding site with the acetylcholinesterase enzyme, and the activity of acetylcholinesterase enzyme disrupt and inhibit esterase (36). The substitutions in these ligands greatly affect the lipophilicity of these ligands (37). Examining the relationship between the structure and activity of this ligand and the activity of the acetylcholinesterase enzyme showed that the presence of aromatic rings, hydrophobic parts, tertiary amines, rotatable bonds, and hydrogen bonds, and other bonds are effective in increasing the inhibitory interaction power of these ligands. It can be concluded that the formation of optimal hydrogen bonds in these compounds to increase hydrophobic interactions is one of the significant factors in inhibiting the various pathways activated by the activity of the acetylcholinesterase enzyme (38). By examining all the amino acids involved in the interactions between the ligands and the enzyme, it was observed that the three amino acids HIS381, TRP385, and GLN527 play a role in the interactions with the acetylcholinesterase enzyme in all ligands through different bonds, indicating the importance of the location of these three amino acids in the enzyme structure. It is acetylcholinesterase. Likewise, the molecular properties, drug similarity, toxicity, absorption, metabolism, secretion, and release of inhibitory ligand compounds were investigated using results from Molsoft, PKCSM, Way2Drug Swiss ADME, and ADMETlab 2.0 servers. The examined ligands demonstrated favorable conditions in terms of solubility coefficient (logS < -4), molecular weight (MW < 500), and octanol/water ratio, indicating the compound’s hydrophilicity (logP < 5) (Table 2). In terms of inhibiting CYPS compounds, they had no inhibitory power and no hepatotoxicity, and they all had intestinal absorption, and in terms of crossing the blood-brain barrier, ligand 1 could cross the blood-brain barrier like the reference compound (39). All selective inhibitory ligands obeyed Lipinski’s five rules. Therefore, based on the characteristics of the therapeutic factors of these compounds and based on the docking score and interaction with the remaining active site of the enzyme and binding ability, it was observed that all of them can be considered possible medicinal compounds in biological systems without any violation of the mentioned characteristics. (Table 2) (40). As a result, they are worth testing for biochemical tests and can be prescribed as a valuable drug for human consumption after the clinical phase processes in the laboratory with the necessary precautions. Although these features are significant in silico studies, the studies in laboratory and clinical conditions on animals must be considered to ensure the correctness of in silico results related to inhibitory ligands.
Conclusion
This study used theoretical and computational methods such as molecular docking to investigate the combination of acetylcholinesterase complex with designed ligands. Based on the investigation and analysis of the docking process done to clarify the mechanism of connection of the herbal medicine with the structure of AChE. The results showed that all these ligands, like donepezil, can interact with HIS381, TRP385, and GLN527 residues of AChE, which all fall in the active site or binding pocket of the active site, which may be critical to inhibiting its activity. The dockings support the hypothesis that these compounds are potentially valuable small molecule ligands for targeting/inhibiting acetylcholinesterases. In fact, according to the binding free energy calculation results, it can be concluded that these ligands can compete with donepezil. It can have a good competitive inhibitory effect by affecting the formation of the acetylcholinesterase/donepezil complex.
On the other hand, the study on the designed ligands showed that with favorable interactions and lower binding energy, they form more stable complexes with acetylcholinesterase and can be proposed as inhibitors competing with donepezil in binding to this enzyme. These results are valuable for designing more non-covalent type inhibitors with high specificity and strong activity because these ligands can be suitable options for conducting experimental investigations to obtain new acetylcholinesterase inhibitor compounds and, as a result, Alzheimer’s treatment. These require laboratory and clinical procedures.
Acknowledgments
The article’s authors thank all the people who helped in different stages of this research.
Funding source
The current research work has no source of funding.
Ethical statement
The authors declare that there is no conflict of interest in the present study.
Author contributions
All authors contribute equally to this research work.
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly and is characterized by loss of memory and other cognitive functions. It is a neurodegenerative disease characterized by decreased cognitive function in patients due to forming Aβ peptides and neurofibrillary tangles (NFT) in the brain (1). AD and other age-related memory disorders have always attracted the attention of researchers worldwide as a slowly progressive disorder characterized by the appearance of neurofibrillary tangles, neural plaques, rapid loss of synapses, degeneration of primary cholinergic neurons, and impaired reasoning. Planning, perception, and thinking are defined. Moreover, it is associated with some metabolic disorders (2,3). This disease becomes a big challenge for the health and social care system and a significant economic burden in the future (4). Therefore, the need to develop new treatments can reduce this risk. Acetylcholinesterase, known as AChE, is an essential enzyme in the family of serine hydrolases that plays a crucial role in memory and cognition (5,6). Cholinesterase is one of the targets used in the design of new drugs for the treatment of AD since the nerve cells that secrete this neurotransmitter substance are among the first cells affected by the pathological changes of AD and are destroyed because the cholinergic neurons located in the basal forebrain, including the neurons that form the basal nucleus form Meinert, which is severely lost in AD. Therefore, it seems that preventing the breakdown of acetylcholine by inhibiting the cholinesterase enzyme can be vital in stabilizing memory and thinking power (7). So far, only seven drugs, capractamine, donepezil, galantamine, huperzine, memantine, rivastigmine, and tacrine (8,9), have been approved by the Food and Drug Administration (FDA) for treating AD. Due to numerous side effects such as hepatotoxicity, gastrointestinal disorders, dizziness, diarrhea, vomiting, nausea, and pharmacokinetic disadvantages, there are many limitations in using these treatment options for AD, a significant reason for discovering new, more effective compounds (10). Growing evidence shows that natural compounds are useful for studying the inhibitory effect on AChE activity. Researchers are constantly trying to find new medicines that, in addition to having better effects, have fewer side effects, and plants have always been essential sources for finding new drugs with acetylcholine enzyme inhibitory effects, which have been of interest. Plants provide materials rich in bioactive compounds, which can be considered a basic strategy for treating various diseases, such as AD (11). Thus, during various studies, several plant chemicals, namely alkaloids, pregnane glycosides (cynancoids), stilbenes, triterpenes (12), ursane (13), and xanthones have shown AChE inhibitory activity. In general, many families of different plants, such as Asteraceae (14), Menispermaceae (15), Malvaceae (16), Zingibraceae (17), and Hypericaceae, have been subjected to various studies, and the extracts and secondary compounds obtained from their different parts have been able to get promising results, to inhibit the activity of the acetylcholinesterase enzyme and to provide researchers with the treatment of AD. The development of new drugs with many clinical applications is needed to reduce the consequences of many diseases. Today, scientists generate large sets of 3D structural data and several leaders and reference therapeutic compounds.


Hence, the drug discovery process requires virtual screening of drugs, molecular docking, and molecular dynamics simulation studies to deal with such a large data set and design new therapeutic compounds. Virtual screening combined with molecular and binding modeling helps to develop new enzyme inhibitors and pharmacophores that bind to receptor sites (18). In this study, natural molecules were collected and identified based on qualitative and quantitative pharmacophoric characteristics and AChE inhibition through databases containing natural compounds and the pharmite server. The analysis results and the best inhibitory compounds were identified for research and subjected to additional analysis and investigation.
Methods
Preparation of Acetylcholinesterase Complex with Donepezil enzyme files and ligand design
This study first prepared the PDB structure (three-dimensional structure) of the Acetylcholinesterase Complex with Donepezil using its access code from the RCSB database. Then, this complex was selected as the leader compound for the virtual pharmacophore search.
Preparation of receptor-ligand for the pharmacophore process
In this step, using the pharmit server, the complex containing the drug-inhibiting receptor was prepared for the pharmacophore process, and the receptor was also prepared through SPDBV.4.1 and MVD software.
Pharmacophore modeling
In this step, inhibitor/receptor files were called by ZINC Pharmer and Pharmit databases and used as a template to find new inhibitory ligands to inhibit the desired 7E3H enzyme. Then, all the inhibitory ligands were retrieved from these databases based on this main inhibitory template called donepezil, and the ligands that had a high similarity to the template ligand were selected for the docking process so that donepezil was confirmed. It searches databases containing natural compounds and finds natural ligands similar to donepezil’s model drug for docking.
Checking the stability of the receptor composition
At this stage, using Iuperd2 software, the sequence structure of the renin enzyme file and its stability level were investigated to ensure stable inhibition with inhibitory ligands (19).
Docking and visualization of ligand/receptor interactions
At this stage, using MVD and AutoDock vina software, the docking process between the receptor and a number of the best inhibitory ligands was selected from among all the selected ligands by the pharmacophore. Finally, ligand/receptor interactions were shown by Discovery Studio software.
Investigating the ADME characteristics of the reference compound and selected superior ligands
At this stage, using some software and servers such as molsoft, PKCSM swiss ADME, and ADMEtlab 2.0, molecular properties and drug similarity, toxicity, absorption, metabolism, secretion, and release of inhibitory ligand compounds were investigated.
Results
Results of preparation of Acetylcholinesterase Complex with Donepezil enzyme files and ligand design
In this study, the PDB structure (three-dimensional structure) of the acetylcholinesterase enzyme compound with the access code (7E3H) was prepared from the RCSB database (20). Then, by studying various articles on the inhibitory compounds of acetylcholinesterase, the donepezil compound, the vital approved drug to inhibit this enzyme, was selected as the leader compound for the virtual pharmacophoric search (Figure 2).
The results of receptor-ligand preparation for the pharmacophore process
At this stage, using SPDV-4.1 and MVD software, the studied ligands and receptor were minimized in terms of energy, and the heteroatoms of the receptor were removed to perform the pharmacophore process. Furthermore, the leader ligand and its receptor, the enzyme acetylcholinesterase, were prepared (Figure 3).
Results of pharmacophore and virtual search
The acetylcholinesterase enzyme, along with its inhibitor Donepezil (7E3H), is listed in the Pharmit database. Essential features for creating pharmacophore models in the desired complex, such as aromatic rings, hydrophobic bonds, hydrogen bond donors, and hydrogen bond acceptors, were analyzed (21). Thus, through the pharmit server and the databases of natural compounds in it, based on the characteristics of the descriptors of the model complex, among more than 88,619 ligands in this server, finally, based on the characteristics and degree of similarity with the model ligand, Donepezil, from the database of natural compounds, six ligands were prepared and selected for further analysis for the molecular docking process, all of which followed Lipinski’s five rules and had good ADMET properties.
The results of investigating the stability of the receptor composition
The results of the IUPred2 software show a stable structure and no disorder in the receptor in the binding sites between the receptor and the studied ligands (Figure 4).
Docking results and display of ligand/receptor interactions
During the docking process, the complexes with the lowest energy were selected from among the results (Table 1).
Then, with the help of different software such as Discovery Studio, all the interactions resulting from docking selected top ligands with acetylcholinesterase enzyme were illustrated. All the essential links participating in these interactions were displayed, and finally, the vital amino acids participating in all the ligands were shown and identified by drawing a thermal diagram (Figure 5).
The results of investigating the ADME characteristics of the reference compound and selected superior ligands
The following results were obtained using molsoft, PKCSM, swissADME, and ADMEtlab 2.0 servers, investigating the molecular properties, drug similarity, toxicity, absorption, metabolism, secretion, and release of inhibitory ligand compounds. According to the obtained results, the obtained ligands all had gastrointestinal absorption. In addition, ligands 1, 3, 4, and 6 had good solubility in fats compared to donepezil, and the obtained ligands followed Lipinski’s principles and had favorable solubility and acceptable pharmaceutical similarity (Table 2).


Discussion
Many efforts have been focused on finding new ligands based on natural compounds that can bind to the active site of enzymes to find stronger reversible inhibitors. Therefore, investigating and predicting the interactions between small molecules and enzyme proteins can be vital for deciphering many biological processes and plays an important role in discovering new drugs with fewer side effects. On the other hand, much evidence has shown that among the types of native and natural plants, the organic parts obtained from the extracts of some different medicinal plants have shown a significant inhibitory effect on AChE. Plants provide materials rich in bioactive compounds, which can be considered a basic strategy for treating various diseases, such as AD (22,23). However, nowadays, laboratory and experimental investigation of anti-enzyme properties of medicinal plants and their effective compounds is time-consuming, expensive, and with the possibility of human error. Therefore, in recent years, the introduction of complementary or alternative methods of experimental methods, such as bioinformatics and computational methods, has received much attention. This research used the pharmacophore process and virtual search in the format database, ZincPharmer. Besides, based on the pharmacophore and structural characteristics of the reference acetylcholinesterase inhibitor compound (donepezil), a large number of natural compound ligands were obtained through virtual search. During the molecular binding process between these ligand compounds, the obtained ligand was done with acetylcholinesterase enzyme, and the best binding energy between the ligands and the receptor was selected from among them (24). Among thousands of natural compounds, finally, the top six ligands based on the reference compound of acetylcholinesterase inhibitor (donepezil) were subsequently selected as a suitable binding model with AChE by using molecular docking methods and through various bioinformatics tools and based on scores. Finally, their interaction with the active site of the AChE enzyme was displayed using the Discovery Studio program (25). In general, many studies have shown therapeutic effects on AD. One study found that a group of plants that have been reported to have the biological effects of the abundance of plants rich in terpene and coumarin compounds are the plants of the genus Ferula. Another study found that the essential oil of Thymus vulgaris or garden thyme, a plant from the mint family, contains phenols such as thymol, carvacrol, simene, linalool, and pinene, whose anti-Alzheimer effect has been investigated, and proven. In other studies, it has been determined that lemon balm, with the scientific name L. Melissa officinalis, is a plant from the mint family used in traditional Iranian medicine in treating a wide range of diseases, including neurological diseases such as amnesia, epilepsy, paralysis, stroke, migraine, and vertigo. Many pharmacological studies show the neuroprotective effects of lemon balm and its main active ingredient (rosmarinic acid). Lemon balm exerts its effects with different mechanisms, including suppressing oxidative stress, inhibiting acetylcholinesterase, stimulating acetylcholine and GABA-A receptors, and inhibiting metalloproteinase-2 and monoamine oxidase enzymes (26). During the results of the docking process, it was shown that ligand 4, which had a higher binding energy than the others (VINA SCORE=-11) and with the acetylcholinesterase enzyme through normal hydrogen interactions with amino acids ARG525, GLN527, ASP404, and also through p-alkyl and alkyl bonds. Through HIS381, ALA397, LEU380, and ARG393, it has established important interactions with the acetylcholinesterase enzyme, most of which established bonds similar to the reference compound with the acetylcholinesterase enzyme (Figures 4, 5) and had a high interaction similarity with the reference ligand. The other top ligand was ligand 2 (VINA SCORE=-10), showing the most structural and interaction similarity with the reference inhibitory ligand. This ligand has three hydrogen bonds, LEU380, GLN527, and ARG525, and two hydrogen/carbon bonds, TYR382. In addition, HIS381 had pi-alkyl interactions,
and PHE531, HIS381, ALA528, ALA397, and a pi-Omid bond through TYR382 (Figures 2-5). Another significant ligand with high similarity to the reference compound in terms of interactions was ligand number 5 (VINASCORE=-10.5). This ligand has four hydrogen bonds, ALA528, TYR382, GLN527, HIS381, and a hydrogen/carbon bond ASP400. In addition, it has pi-alkyl interaction with ALA528 and ALA397, and It has established a sigma-pi bond through TYR382 and a pi-pi-shaped, pi-pi-stacked bond with the amino acid TYR382 (Figure 5).In general, among the six selected ligands, these two ligands, numbers 2 and 5, had the most interaction similarity with the reference ligand, and they probably show a good inhibitory effect, specifically ligand 5 (Figure 4B). Ligand number 2 is obtained from the Asteraceae family. Besides, in a study, it was found that the secondary compounds obtained from different parts of this family of plants, such as the flavonoid compounds found in Silybum marianum, show the potential to inhibit the accumulation of AChE and Aβ peptides (27), revealed by a series of assays, including AChE inhibition assays, as well as in vivo and silico docking studies. Mice treated with silibinin performed better than control mice in various tests. In addition, molecular dynamics simulations indicated that silybinin has a dual inhibitory function against AChE and Aβ peptide accumulation (28). In another study investigating the effect of the methanolic extract of another plant from this family called Phagnalon saxatile, the inhibitory power of various compounds of this extract was shown to inhibit both AChE and BChE enzymes. During the purification of the methanolic extract of this plant, it was found that this extract contains several phenolic compounds, among which luteolin and 3,5-caffeoylquinic acid showed the strongest inhibitory activity against AChE and caffeic acid and luteolin against BChE (29). Furthermore, in another study, chloroform and methanol extracts of Pulicaria stephanocarpa from this family of plants were tested for AChE inhibition, and the results showed that chloroform extract revealed more inhibitory activity (30). Another study tested the ethanolic extract of Artemisia annua, four fractions, and five isolated compounds for AChE inhibitory activity. Two of the four fractions were effective as AChEI inhibitors. However, two of the five isolated compounds - artemisinin (31) and chrysosplantin (32) were the most potent and had the highest AChE inhibitory activity (33). Ligand No. 5 is a plant from the Malvaceae family, and various studies report the effectiveness of different species’ extracts in inhibiting the acetylcholinesterase enzyme. It was observed that the extract showed the effects of inhibiting AChE (34). In another study, the whole plant of Sida rhombifolia Linn was extracted with methanol, ethyl acetate, and n-hexane, and it was found that the n-hexane extract had the highest inhibitory effect on AChE with an inhibition percentage of 68.5% (35). Docking results show that each of these compounds interacts with residues involved in substrate selectivity and catalytic activity, affecting the physicochemical properties and spatial orientation in the binding site with the acetylcholinesterase enzyme, and the activity of acetylcholinesterase enzyme disrupt and inhibit esterase (36). The substitutions in these ligands greatly affect the lipophilicity of these ligands (37). Examining the relationship between the structure and activity of this ligand and the activity of the acetylcholinesterase enzyme showed that the presence of aromatic rings, hydrophobic parts, tertiary amines, rotatable bonds, and hydrogen bonds, and other bonds are effective in increasing the inhibitory interaction power of these ligands. It can be concluded that the formation of optimal hydrogen bonds in these compounds to increase hydrophobic interactions is one of the significant factors in inhibiting the various pathways activated by the activity of the acetylcholinesterase enzyme (38). By examining all the amino acids involved in the interactions between the ligands and the enzyme, it was observed that the three amino acids HIS381, TRP385, and GLN527 play a role in the interactions with the acetylcholinesterase enzyme in all ligands through different bonds, indicating the importance of the location of these three amino acids in the enzyme structure. It is acetylcholinesterase. Likewise, the molecular properties, drug similarity, toxicity, absorption, metabolism, secretion, and release of inhibitory ligand compounds were investigated using results from Molsoft, PKCSM, Way2Drug Swiss ADME, and ADMETlab 2.0 servers. The examined ligands demonstrated favorable conditions in terms of solubility coefficient (logS < -4), molecular weight (MW < 500), and octanol/water ratio, indicating the compound’s hydrophilicity (logP < 5) (Table 2). In terms of inhibiting CYPS compounds, they had no inhibitory power and no hepatotoxicity, and they all had intestinal absorption, and in terms of crossing the blood-brain barrier, ligand 1 could cross the blood-brain barrier like the reference compound (39). All selective inhibitory ligands obeyed Lipinski’s five rules. Therefore, based on the characteristics of the therapeutic factors of these compounds and based on the docking score and interaction with the remaining active site of the enzyme and binding ability, it was observed that all of them can be considered possible medicinal compounds in biological systems without any violation of the mentioned characteristics. (Table 2) (40). As a result, they are worth testing for biochemical tests and can be prescribed as a valuable drug for human consumption after the clinical phase processes in the laboratory with the necessary precautions. Although these features are significant in silico studies, the studies in laboratory and clinical conditions on animals must be considered to ensure the correctness of in silico results related to inhibitory ligands.

Conclusion
This study used theoretical and computational methods such as molecular docking to investigate the combination of acetylcholinesterase complex with designed ligands. Based on the investigation and analysis of the docking process done to clarify the mechanism of connection of the herbal medicine with the structure of AChE. The results showed that all these ligands, like donepezil, can interact with HIS381, TRP385, and GLN527 residues of AChE, which all fall in the active site or binding pocket of the active site, which may be critical to inhibiting its activity. The dockings support the hypothesis that these compounds are potentially valuable small molecule ligands for targeting/inhibiting acetylcholinesterases. In fact, according to the binding free energy calculation results, it can be concluded that these ligands can compete with donepezil. It can have a good competitive inhibitory effect by affecting the formation of the acetylcholinesterase/donepezil complex.
On the other hand, the study on the designed ligands showed that with favorable interactions and lower binding energy, they form more stable complexes with acetylcholinesterase and can be proposed as inhibitors competing with donepezil in binding to this enzyme. These results are valuable for designing more non-covalent type inhibitors with high specificity and strong activity because these ligands can be suitable options for conducting experimental investigations to obtain new acetylcholinesterase inhibitor compounds and, as a result, Alzheimer’s treatment. These require laboratory and clinical procedures.
Acknowledgments
The article’s authors thank all the people who helped in different stages of this research.
Funding source
The current research work has no source of funding.
Ethical statement
In the research work, all ethical processes have been followed.
Conflict of interestThe authors declare that there is no conflict of interest in the present study.
Author contributions
All authors contribute equally to this research work.
Type of Article: Original article |
Subject:
Basic Medical Sciences
Received: 2023/01/25 | Accepted: 2023/06/11 | Published: 2023/07/1
Received: 2023/01/25 | Accepted: 2023/06/11 | Published: 2023/07/1
References
1. Mohammadzadeh Honarvar N, Saedisomeolia A, Abdolahi M, Shayeganrad A, Taheri Sangsari G, Hassanzadeh Rad B, et al. Molecular anti-inflammatory mechanisms of retinoids and carotenoids in Alzheimer's disease: A review of current evidence. J Mol Neurosci. 2017;61(3):289-304. [view at publisher] [DOI] [PMID] [Google Scholar]
2. Waldemar G, Dubois B, Emre M, Georges J, McKeith IG, Rossor M, Scheltens P, Tariska P, Winblad B. Recommendations for the diagnosis and management of Alzheimer's disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14(1):e1-26. [view at publisher] [DOI] [PMID] [Google Scholar]
3. Sugimoto H. Development of anti-Alzheimer's disease drug based on beta-amyloid hypothesis. Yakugaku zasshi: Journal of the Pharmaceutical Society of Japan. 2010;130(4):521-6. [view at publisher] [DOI] [PMID] [Google Scholar]
4. Kennedy DO, Dodd FL, Robertson BC, Okello EJ, Reay JL, Scholey AB, Haskell CF. Monoterpenoid extract of sage (Salvia lavandulaefolia) with cholinesterase inhibiting properties improves cognitive performance and mood in healthy adults. J Psychopharmacol. 2011;25(8):1088-100. [view at publisher] [DOI] [PMID] [Google Scholar]
5. Akhtar MN, Lam KW, Abas F, Ahmad S, Shah SA, Choudhary MI, Lajis NH. New class of acetylcholinesterase inhibitors from the stem bark of Knema laurina and their structural insights. Bioorg Med Chem Lett. 2011;21(13):4097-103. [view at publisher] [DOI] [PMID] [Google Scholar]
6. Xu Y, Colletier JP, Jiang H, Silman I, Sussman JL, Weik M. Induced‐fit or preexisting equilibrium dynamics? Lessons from protein crystallography and MD simulations on acetylcholinesterase and implications for structure‐based drug design. Protein Sci. 2008;17(4):601-5. [view at publisher] [DOI] [PMID] [Google Scholar]
7. Ballard CG, Greig NH, Guillozet-Bongaarts AL, Enz A, Darvesh S. Cholinesterases: roles in the brain during health and disease. Curr Alzheimer Res. 2005;2(3):307-18. [view at publisher] [DOI] [PMID] [Google Scholar]
8. Guevara-Salazar JA, Espinoza-Fonseca M, Beltrán HI, Correa-Basurto J, Quintana Zavala D, Trujillo-Ferrara JG. The electronic influence on the active site-directed inhibition of acetylcholinesterase by N-aryl-substituted succinimides. Journal of the Mexican Chemical Society. 2007;51(4):222-7. [view at publisher] [Google Scholar]
9. Grover A, Shandilya A, Agrawal V, Bisaria VS, Sundar D. Computational evidence to inhibition of human acetyl cholinesterase by withanolide a for Alzheimer treatment. J Biomol Struct Dyn. 2012;29(4):651-62. [view at publisher] [DOI] [PMID] [Google Scholar]
10. Camps P, Formosa X, Galdeano C, Gómez T, Muñoz-Torrero D, Ramírez L, et al. Tacrine-based dual binding site acetylcholinesterase inhibitors as potential disease-modifying anti-Alzheimer drug candidates. Chem Biol Interact. 2010;187(1-3):411-5. [view at publisher] [DOI] [PMID] [Google Scholar]
11. Stefanou V, Matiadis D, Melagraki G, Afantitis A, Athanasellis G, Igglessi-Markopoulou O, et al. Functionalized 4-hydroxy coumarins: novel synthesis, crystal structure and DFT calculations. Molecules. 2011;16(1):384-402. [view at publisher] [DOI] [PMID] [Google Scholar]
12. dos Santos Pisoni D, da Costa JS, Gamba D, Petzhold CL, de Amorim Borges AC, Ceschi MA, et al. Synthesis and AChE inhibitory activity of new chiral tetrahydroacridine analogues from terpenic cyclanones. Eur J Med Chem. 2010;45(2):526-35. [view at publisher] [DOI] [PMID] [Google Scholar]
13. Gurovic MS, Castro MJ, Richmond V, Faraoni MB, Maier MS, Murray AP. Triterpenoids with acetylcholinesterase inhibition from Chuquiraga erinacea D. Don. subsp. erinacea (Asteraceae). Planta Med. 2010;76(6):607-10. [view at publisher] [DOI] [PMID] [Google Scholar]
14. Carpinella MC, Andrione DG, Ruiz G, Palacios SM. Screening for acetylcholinesterase inhibitory activity in plant extracts from Argentina. Phytother Res: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2010;24(2):259-63. [view at publisher] [DOI] [PMID] [Google Scholar]
15. Peng XW, Dong KL. Clinical observation on acupuncture combined with Yizhi Jiannao granules for treatment of Alzheimer's disease. Zhongguo Zhen jiu: Chinese Acupuncture & Moxibustion. 2009;29(4):269-71. [view at publisher] [PMID] [Google Scholar]
16. Mah SH, Teh SS, Ee GC. Anti-inflammatory, anti-cholinergic and cytotoxic effects of Sida rhombifolia. Pharm Biol. 2017;55(1):920-8. [view at publisher] [DOI] [PMID] [Google Scholar]
17. Tappayuthpijarn P, Itharat A, Makchuchit S. Acetylcholinesterase inhibitory activity of Thai traditional nootropic remedy and its herbal ingredients. J Med Assoc Thai: Chotmaihet thangphaet. 2011;94(Suppl7):S183-9. [view at publisher] [PMID] [Google Scholar]
18. de Castro AA, da Cunha EF, Pereira AF, Soares FV, Leal DH, Kuca K, et al. Insights into the drug repositioning applied to the Alzheimer's disease treatment and future perspectives. Curr Alzheimer Res. 2018;15(12):1161-78. [view at publisher] [DOI] [PMID] [Google Scholar]
19. Huang SW, Wang W, Zhang MY, Liu QB, Luo SY, Peng Y, et al. The effect of ethyl acetate extract from persimmon leaves on Alzheimer's disease and its underlying mechanism. Phytomedicine. 2016;23(7):694-704. [view at publisher] [DOI] [PMID] [Google Scholar]
20. Pirmoradi S, Darvishkhadem M, Esmaeillashgarian H. Design of an inhibitory ligand based on interleukin 6 receptor for disruption and inhibition of interleukin 6-dependent inflammatory signaling pathway in Covid-19 patients. Yafte. 2021;23:344-55. [view at publisher] [Google Scholar]
21. Erdős G, Dosztányi Z. Analyzing protein disorder with IUPred2A. Curr Protoc Bioinformatics. Current Protocols in Bioinformatics. 2020;70(1):e99. [view at publisher] [DOI] [PMID] [Google Scholar]
22. Refsgaard HH, Jensen BF, Brockhoff PB, Padkjær SB, Guldbrandt M, Christensen MS. In silico prediction of membrane permeability from calculated molecular parameters. J Med Chem. 2005;48(3):805-11. [view at publisher] [DOI] [PMID] [Google Scholar]
23. Debnath AK. Pharmacophore mapping of a series of 2, 4-diamino-5-deazapteridine inhibitors of Mycobacterium avium complex dihydrofolate reductase. J Med Chem. 2002;45(1):41-53. [view at publisher] [DOI] [PMID] [Google Scholar]
24. Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45(12):2615-23. [view at publisher] [DOI] [PMID] [Google Scholar]
25. Boonyaketgoson S, Rukachaisirikul V, Phongpaichit S, Trisuwan K. Naphthoquinones from the leaves of Rhinacanthus nasutus having acetylcholinesterase inhibitory and cytotoxic activities. Fitoterapia. 2018;124:206-10. [view at publisher] [DOI] [PMID] [Google Scholar]
26. Ritchie TJ, Ertl P, Lewis R. The graphical representation of ADME-related molecule properties for medicinal chemists. Drug Discov Today. 2011;16(1-2):65-72. [view at publisher] [DOI] [PMID] [Google Scholar]
27. Pirmoradi S, Jafari H. Design of Immunosuppressive Structure Based on Spike Protein (s) virus Corona. J Arak Uni Med Sci. 2021;24(4):596-615. [view at publisher] [DOI] [Google Scholar]
28. Seniya C, Khan GJ, Misra R, Vyas V, Kaushik S. In-silico modelling and identification of a possible inhibitor of H1N1 virus. Asian Pacific Journal of Tropical Disease. 2014;4(suppl1):S467-76. [view at publisher] [DOI] [Google Scholar]
29. de Castro AA, da Cunha EF, Pereira AF, Soares FV, Leal DH, Kuca K, et al. Insights into the drug repositioning applied to the Alzheimer's disease treatment and future perspectives. Curr Alzheimer Res. 2018;15(12):1161-78. [view at publisher] [DOI] [PMID] [Google Scholar]
30. Wilkinson D, Doody R, Helme R, Taubman K, Mintzer J, Kertesz A, et al. Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology. 2003;61(4):479-86. [view at publisher] [DOI] [PMID] [Google Scholar]
31. Conforti F, Rigano D, Formisano C, Bruno M, Loizzo MR, Menichini F, et al. Metabolite profile and in vitro activities of Phagnalon saxatile (L.) Cass. relevant to treatment of Alzheimer's disease. J Enzyme Inhib Med Chem. 2010;25(1):97-104. [view at publisher] [DOI] [PMID] [Google Scholar]
32. Duan S, Guan X, Lin R, Liu X, Yan Y, Lin R, Zhang T, Chen X, Huang J, Sun X, Li Q. Silibinin inhibits acetylcholinesterase activity and amyloid β peptide aggregation: a dual-target drug for the treatment of Alzheimer's disease. Neurobiol Aging. 2015;36(5):1792-807. [view at publisher] [DOI] [PMID] [Google Scholar]
33. Bakthir H, Ali NA, Arnold N, Teichert A, Wessjohann L. Anticholinesterase activity of endemic plant extracts from Soqotra. Afr J Tradit Complement Altern Med. 2011;8(3):296-9. [view at publisher] [DOI] [PMID] [Google Scholar]
34. Chougouo RD, Nguekeu YM, Dzoyem JP, Awouafack MD, Kouamouo J, Tane P, et al. Anti-inflammatory and acetylcholinesterase activity of extract, fractions and five compounds isolated from the leaves and twigs of Artemisia annua growing in Cameroon. Springerplus. 2016;5(1):1-7. [view at publisher] [DOI] [PMID] [Google Scholar]
35. Zhao Y, Dou J, Wu T, Aisa HA. Investigating the antioxidant and acetylcholinesterase inhibition activities of Gossypium herbaceam. Molecules. 2013;18(1):951-62. [view at publisher] [DOI] [PMID] [Google Scholar]
36. Mah SH, Teh SS, Ee GC. Anti-inflammatory, anti-cholinergic and cytotoxic effects of Sida rhombifolia. Pharm Biol. 2017;55(1):920-8. [view at publisher] [DOI] [PMID] [Google Scholar]
37. Hendrayani SF, Al-Harbi B, Al-Ansari MM, Silva G, Aboussekhra A. The inflammatory/cancer-related IL-6/STAT3/NF-κB positive feedback loop includes AUF1 and maintains the active state of breast myofibroblasts. Oncotarget. 2016;7(27):41974. [view at publisher] [DOI] [PMID] [Google Scholar]
38. Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clinical immunology. 2020;214:108393. [view at publisher] [DOI] [PMID] [Google Scholar]
39. Mohanty SK, Satapathy A, Naidu MM, Mukhopadhyay S, Sharma S, Barton LM, et al. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19)-anatomic pathology perspective on current knowledge. Diagnostic pathology. 2020;15(1):1-7. [view at publisher] [DOI] [PMID] [Google Scholar]
40. Pirmoradi S. In-silico Designing of Immunogenic Construct Based on Peptide Epitopes Using Immuno-informatics Tools Against Tuberculosis. Iran J Med Microbiol. 2022;16(6):506-19. [view at publisher] [DOI] [PMID] [Google Scholar]
41. Iranshahi M, Javadi B. Neurological and Neuroprotective effects of Melissa officinalis L. Navid No. 2019;22(69):60-71. [view at publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





