Volume 18, Issue 6 (Nov-Dec 2024)
mljgoums 2024, 18(6): 36-40 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rasannezhad F, Abbassi Daloii A, Ziaolhagh J, Abdi A. The effect of aerobic exercise and psilocybin following methamphetamine induction on the gene expression of certain semaphorins in female Wistar rats. mljgoums 2024; 18 (6) :36-40
URL: http://mlj.goums.ac.ir/article-1-1807-en.html
URL: http://mlj.goums.ac.ir/article-1-1807-en.html
1- Department of Sport Science, Ayatollah Amoli Branch, Islamic Azad University, Amol, Iran
2- Department of Sport Science, Ayatollah Amoli Branch, Islamic Azad University, Amol, Iran ,abbasi.daloii@gmail.com
3- Department of Sport Science, Shahrood Branch, Islamic Azad University, Shahrood, Iran
2- Department of Sport Science, Ayatollah Amoli Branch, Islamic Azad University, Amol, Iran ,
3- Department of Sport Science, Shahrood Branch, Islamic Azad University, Shahrood, Iran
Full-Text [PDF 418 kb]
(1700 Downloads)
| Abstract (HTML) (4666 Views)
Discussion
In the present study, the average expression of semaphorin-3A, 4D, and 7A genes in methamphetamine-consuming rats showed a significant increase. Methamphetamine can act as a vasoconstrictor and decrease striatal and cortical blood flow through the dopamine D2 receptor (25). Like alcohol, methamphetamine can also cause long-term damage through mitochondrial dysfunction and increased production of ROS and nitric oxide (26). Another result of the research was the reduction in the average gene expression of semaphorins 3A, 4D, and 7A as a result of performing aerobic exercise alone and in combination with psilocybin in rats consuming methamphetamine. Studies have shown that Sema3A signaling causes a local increase in H2O2 in the dorsal root growth cone of the nerve ganglion through the activation of MICAL1 and MICAL3 (27), with another protein, p53 that is a motor suppressor protein, possibly playing an important role in the induction of class 3 semaphorins, especially Sema3B. In research, it has been shown that high expression of Sema3B, in the presence or even absence of p53, can have an apoptotic effect on cancer cells. It has been shown that Sema3B induces apoptosis in these cells through the activation of the caspase-3 enzyme (28). Moretti and his colleagues stated in their research that Sema3A signaling controls apoptosis through Fas (CD95) by transferring Fas into lipid rafts (29). In the study by Fazelzadeh, it was shown that four weeks of voluntary exercise caused a significant decrease in the concentration of H2O2 and Sema3B, and apoptosis in the hippocampus of diabetic rats (30). Sports training has a positive effect on cognitive function and facilitates neurological rehabilitation after brain injury (31). Van Praag stated in his research that voluntary running on a treadmill causes an increase of 3-4 times, or even more, in the production and survival of new nerve cells in the dentate gyrus of the hippocampus (32). Regular exercise can probably play a role in this process through the adaptations it creates in the activity and expression of some influential factors in regulating apoptosis (33,34). The results of some studies showed that intense periodic training reduced the increased expression of Sema3A in the skeletal muscles of old rats (35). As the present study showed, exercise activity decreased the increased concentration of Sema3B protein in the cerebral cortex of rats consuming methamphetamine. Functional brain regions responsible for processing social inclusion and exclusion are located primarily in the insula, and substance abuse directly damages the neural structures of the insula (36). At the same time, methamphetamine abuse leads to an imbalance in the dopaminergic system, where dopamine type 1 receptor signaling in the ventral tegmental area mediates complex social behavior and the availability of striatal D2/3 dopamine receptors (37). Aerobic exercise promotes the expression of brain-derived neurotrophic factor and other neurotrophic factors that support synaptic plasticity and neuronal survival. This upregulation can counteract the synaptic dysfunction caused by methamphetamine. The modulation of semaphorin gene expression by these neurotrophic factors could help in restoring synaptic function and structural integrity in the brain regions affected by methamphetamine (38). Aerobic exercise has anti-inflammatory effects, reducing the levels of pro-inflammatory cytokines and enhancing the expression of anti-inflammatory cytokines. Since semaphorins also play roles in immune modulation, exercise-induced changes in semaphorin expression could contribute to a reduction in neuroinflammation, thereby protecting neural cells from methamphetamine-induced damage (39). Another result of the present study was the decrease in the mean expression of semaphorins 3A, 4D, and 7A due to the use of psilocybin and the combination of exercise with psilocybin in rats consuming methamphetamine. In recent years, there has been scientific reconsideration of the potential use of psilocybin and other psychoactive substances to treat psychiatric disorders, particularly mood disorders, anxiety, and addiction (40). All symptoms were described as transient, and no patient required specific drug treatment (41). The most essential pharmacological property that psilocybin showed in all trials was the rapid onset of the alleviated effect. This effect can be ameliorated when combined with traditional antidepressant treatment, which has a long latency (42). The antidepressant effects of psilocybin appear biological and context-dependent (19). These natural processes include cell proliferation, increased synaptic connectivity, and anti-inflammatory effects (43). Psilocybin facilitates periodic behavioral flexibility, in which exploration of a non-home environment reduces anxiety during future investigation of a novel environment (19). The more sustained therapeutic effects of a single dose of psilocybin compared to ketamine in an experimental system support the idea that serotonin 5-HT2A receptor-directed therapeutic strategies may be superior to ketamine-based treatments in the depression clinic. In addition, psilocybin has regulatory effects on methamphetamine-induced alterations of behavior in rats via dopamine 2 receptor-mediated signal regulation of extracellular signal-regulated kinase phosphorylation (23). Psilocybin promotes neuroplasticity and synaptogenesis by activating serotonin 5-HT2A receptors. This activation leads to the upregulation of immediate early genes involved in synaptic growth and plasticity. Given that semaphorins are integral to synaptic formation and guidance, psilocybin-induced neuroplasticity could involve modulation of semaphorin gene expression, enhancing synaptic connectivity and repair in methamphetamine-affected regions (38). Similar to aerobic exercise, psilocybin has been found to possess anti-inflammatory properties. By reducing neuroinflammation, psilocybin may alter the expression of semaphorin genes involved in immune responses, thus protecting neural tissues from inflammatory damage induced by methamphetamine. Psilocybin impacts the limbic system and other brain areas involved in stress and emotion regulation. Semaphorins are known to play roles in neural circuit development in these regions. Psilocybin-induced modulation of semaphorin expression could therefore contribute to improved emotional regulation and stress resilience in individuals recovering from methamphetamine abuse (39). The interplay between aerobic exercise and psilocybin in modulating semaphorin gene expression offers a promising avenue for mitigating methamphetamine-induced neurotoxicity. Aerobic exercise supports neuroprotection and synaptic function through metabolic and anti-inflammatory pathways, while psilocybin enhances neuroplasticity and reduces neuroinflammation. Both interventions, by modulating semaphorin gene expression, could provide synergistic benefits in restoring neural health and function in the context of methamphetamine abuse. According to the discussed cases, the beneficial effects of exercise and psilocybin on the reduction of semaphorins 3A, 4D, and 7A were evident in rats consuming methamphetamine, and the best results were obtained when they were used simultaneously, which shows the synergistic effect of exercise and psilocybin.
Conclusion
In general, the results of the present research indicate that exercise training and psilocybin in rats using methamphetamine led to a decrease in semaphorins 3A, 4D, and 7A in the cortex of female rats. The best results were obtained in the combined group of exercise and psilocybin, which shows the synergistic effect of these two interventions. Nevertheless, the current research was associated with limitations, such as the use of female rats and the short duration of the interventions, which requires additional research for better results.
Acknowledgement
This article is taken from the thesis of the doctoral course in sports physiology, approved by Islamic Azad University, Ayatollah Amoly Branch. The authors wish to thank all the people who helped us in the progress of the work.
Funding sources
This article is extracted from a Ph.D. thesis at the Ayatollah Amoli Branch of Islamic Azad University and was personally funded by the authors.
Ethical statement
The code of ethics for the current research at Islamic Azad University, Ayatollah Amoli Branch, was reviewed and approved under the principle of ethics IR.IAU.AMOL.REC.1401.104.
Conflicts of interest
The authors have no conflicts of interest regarding the presented results.
Author contributions
FR: Original draft, Methodology. AAD: Writing, Review and Editing, Project Management. JZ: Methodology. AA: Analysis.
Full-Text: (1226 Views)
Introduction
Methamphetamine abuse is a significant public health concern worldwide due to its strong addictive properties (1). It interrupts the reabsorption of dopamine and other single amine neurotransmitters and facilitates the release of these single amines into the synaptic space (2). Studies show that this drug decreases the ability of stem cells to reproduce and self-regenerate in specific parts of the brain, alters their differentiation pathways from normal to abnormal, and impacts the processes of cell formation, growth, and differentiation into stem or neural precursors (3). Recently, microRNAs have been identified to play critical roles in various cellular processes. The expression levels of certain miRNAs are altered after methamphetamine administration, which may affect the transcription of target genes that regulate methamphetamine toxicity or addiction (1). One of the targets of microRNAs is axon guidance molecules, such as semaphorins, which have been shown to contribute to the development of drug reward and addiction (4). Changes in SEMA3A are negatively correlated with miRNAs, suggesting that SEMA3A expression may be regulated by miRNAs in methamphetamine sensitivity (5). Semaphorin 7A (Sema7A) is linked to the plasma membrane through a glycophosphatidylinositol anchor. Some membrane-bound semaphorins can be proteolytically cleaved to produce soluble proteins (6). Semaphorin signaling is primarily mediated through plexin receptors and leads to changes in the cytoskeleton and adhesion apparatus that regulate cell morphology (6,7). Besides plexins and neuropilins, other molecules act as receptors for some semaphorins (8), such as CD72 and T-cell immunoglobulin and mucin domain proteins. These interact with Sema 4D (CD100) and Sema 4A, respectively, in the immune system (9). Integrins also act as transmitters of Sema7A signals in the nervous and immune systems (10). Semaphorin 4D participates in mast cell functions, B lymphocyte functions, and T-cell-mediated immunity. It causes inhibitory synapse formation and acts as an axon guidance factor, with somatic and dendritic inhibitory synapses responding equally to Sema 4D signaling. Semaphorin 7A plays a role in T cell-macrophage communication (11). Sema3 is expressed by activated T cells and dendritic cells (DCs). Sema3A’s receptor, plexin A1, is expressed at low or undetectable levels in other immune cells, such as macrophages, B cells, and T cells. Studies using RNA interference have shown that plexin A1 is involved in communication between T cells and DCs and causes T cell activation by DCs. Semaphorin 3A is associated with immunosuppressive roles and functions of dendritic cells (11). In the nervous system, semaphorins play roles in either repulsion or attraction of axons toward target tissues (12). On the other hand, sports activity has been shown to exert non-invasive and non-pharmacological protective effects against neuromuscular diseases and disabilities. Exercise is crucial for maintaining synaptic function and structure and for the recovery of damaged neurons (13). Today, exercise is considered an essential factor in the mental stability of people, which can have positive effects on people's behavior. Aerobic exercise significantly increases the length of nerve terminal branches and helps maintain the standard size of the endplate (14). It also affects peripheral nerves and neuromuscular junctions by stimulating the expression of growth factors, increasing mitochondrial biogenesis, and enhancing the speed and quantity of axonal transmission (15). Recently, psilocybin (4-phosphoryloxy-N, N-dimethyltryptamine), a natural hallucinogen and primary compound in umbelliferae, has shown significant effects (16,17). After consumption, psilocybin is metabolized into psilocin, which has psychoactive properties. Short-term use of psilocybin has proven effective in treating borderline or bipolar personality disorders, depression, and migraines (18). The appropriate dose for most individuals ranges from 1 to 3.5 grams of dried mushrooms or 10 to 15 grams of fresh mushrooms. Psilocybin mushrooms can sometimes increase heart rate and blood pressure (19). Psilocybin produces various physical and psychological symptoms by stimulating the sympathetic nervous system. As with many psychoactive substances, the effects of psychedelic mushrooms are subjective and can vary considerably between individuals (20). A study by Gotvaldová et al. showed that a single high dose of psilocybin could cause long-lasting changes in personality (21). To date, the precise role of psilocybin and its effects on semaphorins have not been fully determined. Furthermore, the potential synergistic effects of psilocybin and exercise remain unclear. Therefore, this study seeks to investigate the impact of aerobic exercise and psilocybin, in conjunction with methamphetamine induction, on the gene expression of certain cerebral cortex semaphorins in female Wistar rats.
Methods
Rats for this experimental research were obtained from Shahrood University of Medical Sciences. The weight of the rats ranged from 155 to 180 grams. First, 40 female rats were randomly divided into five groups of eight: the first group (Control group (C)), the second group (Methamphetamine group (A)), the third group (Methamphetamine + aerobic group (AA)), the fourth group (Methamphetamine + psilocybin group (AP)), and the fifth group (Methamphetamine + aerobic + psilocybin group (AAP)). The code of ethics for the current research at Islamic Azad University - Ayatollah Amoli Branch was reviewed and approved under the ethical principles IR.IAU.AMOL.REC.1401.104.
The doses of methamphetamine and psilocybin were chosen based on previous studies. Methamphetamine was injected intraperitoneally at a dose of 15 mg every 12 hours for four days (22), and psilocybin was administered intraperitoneally at a dose of 1 mg/kg (19,23).
The training program consisted of an 8-week running regimen with increasing intensity. The total running time was gradually increased from 20 minutes to 30 minutes, and the maximum daily speed was increased from 20 m/min to 25 m/min. Starting in the fourth week, a 5% slope was introduced. Exercises were performed between 8-10 AM. To evaluate the training effect, VO2max was equalized among the rats, and comparisons were made between the groups (24).
To confirm the creation of the methamphetamine-addicted rat model more accurately, behavioral data were also used. For this purpose, Y-maze tests were conducted (Figure 1 and 2)
After eight weeks of applying the independent variables, all samples, under identical conditions and in baseline conditions (48 hours after the last training session), were anesthetized via intraperitoneal injection of a combination of ketamine (60 mg/kg) and xylazine (5 mg/kg). To examine the expression of the target genes, the real-time PCR method (Step One model, made in Italy) was employed. The sequence of primers is shown in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control.
To compare the groups, a one-way analysis of variance and Tukey's post hoc test were used. All analyses were performed using SPSS V.23 statistical software, and results were considered statistically significant at P < 0.05.
Results
The results in the table show that weight changes from the first to the eighth week were less in groups that exercised compared to the other groups. In addition, the VO2max values indicate the effectiveness of the exercise, as the groups that exercised showed higher VO2max values (Table 2).
Expression of Semaphorin 3A gene in cerebral cortex
Based on the findings, the mean expression of the semaphorin 3A gene in the cerebral cortex of the A group increased significantly compared to the C group (p < 0.0001). The AA (p = 0.008), AP (p = 0.012), and AAP (p < 0.0001) groups showed a significant decrease compared to the A group. The AAP group exhibited a considerable decline compared to the AA (p = 0.046) and AP (p = 0.031) groups (Table 3).
Expression of semaphorin 4A gene in cerebral cortex
The findings showed that the mean expression of the semaphorin 4A gene in the cerebral cortex of the A group increased significantly compared to the C group (p = 0.001). The AA (p = 0.001), AP (p = 0.005), and AAP (p < 0.0001) groups showed a significant decrease compared to the A group (Table 3).
Expression of semaphorin 7A gene in cerebral cortex
Based on these findings, the mean expression of the semaphorin 7A gene in the cerebral cortex of the A group increased significantly compared to the C group (p < 0.0001). The AAP group showed a significant decrease compared to the A (p = 0.001) and AP (p = 0.007) groups (Table 3).
Behavioral data
The comparison between before and after methamphetamine induction in the variable of the total number of arm entries showed a significant difference (Figure 1; P < 0.0001). In addition, the comparison before and after methamphetamine induction showed a significant difference in the non-repetitive interval count variable (Figure 2; P < 0.0001).
Methamphetamine abuse is a significant public health concern worldwide due to its strong addictive properties (1). It interrupts the reabsorption of dopamine and other single amine neurotransmitters and facilitates the release of these single amines into the synaptic space (2). Studies show that this drug decreases the ability of stem cells to reproduce and self-regenerate in specific parts of the brain, alters their differentiation pathways from normal to abnormal, and impacts the processes of cell formation, growth, and differentiation into stem or neural precursors (3). Recently, microRNAs have been identified to play critical roles in various cellular processes. The expression levels of certain miRNAs are altered after methamphetamine administration, which may affect the transcription of target genes that regulate methamphetamine toxicity or addiction (1). One of the targets of microRNAs is axon guidance molecules, such as semaphorins, which have been shown to contribute to the development of drug reward and addiction (4). Changes in SEMA3A are negatively correlated with miRNAs, suggesting that SEMA3A expression may be regulated by miRNAs in methamphetamine sensitivity (5). Semaphorin 7A (Sema7A) is linked to the plasma membrane through a glycophosphatidylinositol anchor. Some membrane-bound semaphorins can be proteolytically cleaved to produce soluble proteins (6). Semaphorin signaling is primarily mediated through plexin receptors and leads to changes in the cytoskeleton and adhesion apparatus that regulate cell morphology (6,7). Besides plexins and neuropilins, other molecules act as receptors for some semaphorins (8), such as CD72 and T-cell immunoglobulin and mucin domain proteins. These interact with Sema 4D (CD100) and Sema 4A, respectively, in the immune system (9). Integrins also act as transmitters of Sema7A signals in the nervous and immune systems (10). Semaphorin 4D participates in mast cell functions, B lymphocyte functions, and T-cell-mediated immunity. It causes inhibitory synapse formation and acts as an axon guidance factor, with somatic and dendritic inhibitory synapses responding equally to Sema 4D signaling. Semaphorin 7A plays a role in T cell-macrophage communication (11). Sema3 is expressed by activated T cells and dendritic cells (DCs). Sema3A’s receptor, plexin A1, is expressed at low or undetectable levels in other immune cells, such as macrophages, B cells, and T cells. Studies using RNA interference have shown that plexin A1 is involved in communication between T cells and DCs and causes T cell activation by DCs. Semaphorin 3A is associated with immunosuppressive roles and functions of dendritic cells (11). In the nervous system, semaphorins play roles in either repulsion or attraction of axons toward target tissues (12). On the other hand, sports activity has been shown to exert non-invasive and non-pharmacological protective effects against neuromuscular diseases and disabilities. Exercise is crucial for maintaining synaptic function and structure and for the recovery of damaged neurons (13). Today, exercise is considered an essential factor in the mental stability of people, which can have positive effects on people's behavior. Aerobic exercise significantly increases the length of nerve terminal branches and helps maintain the standard size of the endplate (14). It also affects peripheral nerves and neuromuscular junctions by stimulating the expression of growth factors, increasing mitochondrial biogenesis, and enhancing the speed and quantity of axonal transmission (15). Recently, psilocybin (4-phosphoryloxy-N, N-dimethyltryptamine), a natural hallucinogen and primary compound in umbelliferae, has shown significant effects (16,17). After consumption, psilocybin is metabolized into psilocin, which has psychoactive properties. Short-term use of psilocybin has proven effective in treating borderline or bipolar personality disorders, depression, and migraines (18). The appropriate dose for most individuals ranges from 1 to 3.5 grams of dried mushrooms or 10 to 15 grams of fresh mushrooms. Psilocybin mushrooms can sometimes increase heart rate and blood pressure (19). Psilocybin produces various physical and psychological symptoms by stimulating the sympathetic nervous system. As with many psychoactive substances, the effects of psychedelic mushrooms are subjective and can vary considerably between individuals (20). A study by Gotvaldová et al. showed that a single high dose of psilocybin could cause long-lasting changes in personality (21). To date, the precise role of psilocybin and its effects on semaphorins have not been fully determined. Furthermore, the potential synergistic effects of psilocybin and exercise remain unclear. Therefore, this study seeks to investigate the impact of aerobic exercise and psilocybin, in conjunction with methamphetamine induction, on the gene expression of certain cerebral cortex semaphorins in female Wistar rats.
Methods
Rats for this experimental research were obtained from Shahrood University of Medical Sciences. The weight of the rats ranged from 155 to 180 grams. First, 40 female rats were randomly divided into five groups of eight: the first group (Control group (C)), the second group (Methamphetamine group (A)), the third group (Methamphetamine + aerobic group (AA)), the fourth group (Methamphetamine + psilocybin group (AP)), and the fifth group (Methamphetamine + aerobic + psilocybin group (AAP)). The code of ethics for the current research at Islamic Azad University - Ayatollah Amoli Branch was reviewed and approved under the ethical principles IR.IAU.AMOL.REC.1401.104.
The doses of methamphetamine and psilocybin were chosen based on previous studies. Methamphetamine was injected intraperitoneally at a dose of 15 mg every 12 hours for four days (22), and psilocybin was administered intraperitoneally at a dose of 1 mg/kg (19,23).
The training program consisted of an 8-week running regimen with increasing intensity. The total running time was gradually increased from 20 minutes to 30 minutes, and the maximum daily speed was increased from 20 m/min to 25 m/min. Starting in the fourth week, a 5% slope was introduced. Exercises were performed between 8-10 AM. To evaluate the training effect, VO2max was equalized among the rats, and comparisons were made between the groups (24).
To confirm the creation of the methamphetamine-addicted rat model more accurately, behavioral data were also used. For this purpose, Y-maze tests were conducted (Figure 1 and 2)
After eight weeks of applying the independent variables, all samples, under identical conditions and in baseline conditions (48 hours after the last training session), were anesthetized via intraperitoneal injection of a combination of ketamine (60 mg/kg) and xylazine (5 mg/kg). To examine the expression of the target genes, the real-time PCR method (Step One model, made in Italy) was employed. The sequence of primers is shown in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control.
To compare the groups, a one-way analysis of variance and Tukey's post hoc test were used. All analyses were performed using SPSS V.23 statistical software, and results were considered statistically significant at P < 0.05.
Results
The results in the table show that weight changes from the first to the eighth week were less in groups that exercised compared to the other groups. In addition, the VO2max values indicate the effectiveness of the exercise, as the groups that exercised showed higher VO2max values (Table 2).
Expression of Semaphorin 3A gene in cerebral cortex
Based on the findings, the mean expression of the semaphorin 3A gene in the cerebral cortex of the A group increased significantly compared to the C group (p < 0.0001). The AA (p = 0.008), AP (p = 0.012), and AAP (p < 0.0001) groups showed a significant decrease compared to the A group. The AAP group exhibited a considerable decline compared to the AA (p = 0.046) and AP (p = 0.031) groups (Table 3).
Expression of semaphorin 4A gene in cerebral cortex
The findings showed that the mean expression of the semaphorin 4A gene in the cerebral cortex of the A group increased significantly compared to the C group (p = 0.001). The AA (p = 0.001), AP (p = 0.005), and AAP (p < 0.0001) groups showed a significant decrease compared to the A group (Table 3).
Expression of semaphorin 7A gene in cerebral cortex
Based on these findings, the mean expression of the semaphorin 7A gene in the cerebral cortex of the A group increased significantly compared to the C group (p < 0.0001). The AAP group showed a significant decrease compared to the A (p = 0.001) and AP (p = 0.007) groups (Table 3).
Behavioral data
The comparison between before and after methamphetamine induction in the variable of the total number of arm entries showed a significant difference (Figure 1; P < 0.0001). In addition, the comparison before and after methamphetamine induction showed a significant difference in the non-repetitive interval count variable (Figure 2; P < 0.0001).
|
Table 1. The primer pattern of semaphorins
 Table 2. The mean and standard deviation of the weight (gr) and VO2max (ml/kg/min) of rats of different groups  Table 3. The mean and standard deviation of semaphorins gene expression in different research groups  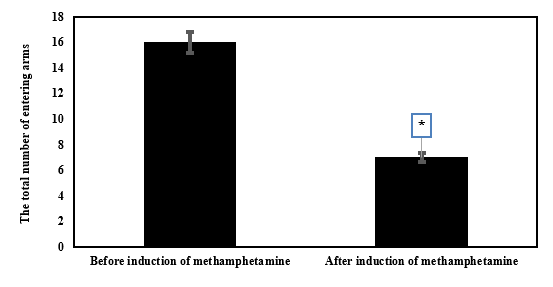 Figure 1. The mean and standard deviation of the total number of arm entries before and after methamphetamine induction. *Significant decrease compared to before methamphetamine induction |
.PNG) Figure 2. The mean and standard deviation of the non-repetitive frequency before and after methamphetamine induction. *Significant decrease compared to before methamphetamine induction |
Discussion
In the present study, the average expression of semaphorin-3A, 4D, and 7A genes in methamphetamine-consuming rats showed a significant increase. Methamphetamine can act as a vasoconstrictor and decrease striatal and cortical blood flow through the dopamine D2 receptor (25). Like alcohol, methamphetamine can also cause long-term damage through mitochondrial dysfunction and increased production of ROS and nitric oxide (26). Another result of the research was the reduction in the average gene expression of semaphorins 3A, 4D, and 7A as a result of performing aerobic exercise alone and in combination with psilocybin in rats consuming methamphetamine. Studies have shown that Sema3A signaling causes a local increase in H2O2 in the dorsal root growth cone of the nerve ganglion through the activation of MICAL1 and MICAL3 (27), with another protein, p53 that is a motor suppressor protein, possibly playing an important role in the induction of class 3 semaphorins, especially Sema3B. In research, it has been shown that high expression of Sema3B, in the presence or even absence of p53, can have an apoptotic effect on cancer cells. It has been shown that Sema3B induces apoptosis in these cells through the activation of the caspase-3 enzyme (28). Moretti and his colleagues stated in their research that Sema3A signaling controls apoptosis through Fas (CD95) by transferring Fas into lipid rafts (29). In the study by Fazelzadeh, it was shown that four weeks of voluntary exercise caused a significant decrease in the concentration of H2O2 and Sema3B, and apoptosis in the hippocampus of diabetic rats (30). Sports training has a positive effect on cognitive function and facilitates neurological rehabilitation after brain injury (31). Van Praag stated in his research that voluntary running on a treadmill causes an increase of 3-4 times, or even more, in the production and survival of new nerve cells in the dentate gyrus of the hippocampus (32). Regular exercise can probably play a role in this process through the adaptations it creates in the activity and expression of some influential factors in regulating apoptosis (33,34). The results of some studies showed that intense periodic training reduced the increased expression of Sema3A in the skeletal muscles of old rats (35). As the present study showed, exercise activity decreased the increased concentration of Sema3B protein in the cerebral cortex of rats consuming methamphetamine. Functional brain regions responsible for processing social inclusion and exclusion are located primarily in the insula, and substance abuse directly damages the neural structures of the insula (36). At the same time, methamphetamine abuse leads to an imbalance in the dopaminergic system, where dopamine type 1 receptor signaling in the ventral tegmental area mediates complex social behavior and the availability of striatal D2/3 dopamine receptors (37). Aerobic exercise promotes the expression of brain-derived neurotrophic factor and other neurotrophic factors that support synaptic plasticity and neuronal survival. This upregulation can counteract the synaptic dysfunction caused by methamphetamine. The modulation of semaphorin gene expression by these neurotrophic factors could help in restoring synaptic function and structural integrity in the brain regions affected by methamphetamine (38). Aerobic exercise has anti-inflammatory effects, reducing the levels of pro-inflammatory cytokines and enhancing the expression of anti-inflammatory cytokines. Since semaphorins also play roles in immune modulation, exercise-induced changes in semaphorin expression could contribute to a reduction in neuroinflammation, thereby protecting neural cells from methamphetamine-induced damage (39). Another result of the present study was the decrease in the mean expression of semaphorins 3A, 4D, and 7A due to the use of psilocybin and the combination of exercise with psilocybin in rats consuming methamphetamine. In recent years, there has been scientific reconsideration of the potential use of psilocybin and other psychoactive substances to treat psychiatric disorders, particularly mood disorders, anxiety, and addiction (40). All symptoms were described as transient, and no patient required specific drug treatment (41). The most essential pharmacological property that psilocybin showed in all trials was the rapid onset of the alleviated effect. This effect can be ameliorated when combined with traditional antidepressant treatment, which has a long latency (42). The antidepressant effects of psilocybin appear biological and context-dependent (19). These natural processes include cell proliferation, increased synaptic connectivity, and anti-inflammatory effects (43). Psilocybin facilitates periodic behavioral flexibility, in which exploration of a non-home environment reduces anxiety during future investigation of a novel environment (19). The more sustained therapeutic effects of a single dose of psilocybin compared to ketamine in an experimental system support the idea that serotonin 5-HT2A receptor-directed therapeutic strategies may be superior to ketamine-based treatments in the depression clinic. In addition, psilocybin has regulatory effects on methamphetamine-induced alterations of behavior in rats via dopamine 2 receptor-mediated signal regulation of extracellular signal-regulated kinase phosphorylation (23). Psilocybin promotes neuroplasticity and synaptogenesis by activating serotonin 5-HT2A receptors. This activation leads to the upregulation of immediate early genes involved in synaptic growth and plasticity. Given that semaphorins are integral to synaptic formation and guidance, psilocybin-induced neuroplasticity could involve modulation of semaphorin gene expression, enhancing synaptic connectivity and repair in methamphetamine-affected regions (38). Similar to aerobic exercise, psilocybin has been found to possess anti-inflammatory properties. By reducing neuroinflammation, psilocybin may alter the expression of semaphorin genes involved in immune responses, thus protecting neural tissues from inflammatory damage induced by methamphetamine. Psilocybin impacts the limbic system and other brain areas involved in stress and emotion regulation. Semaphorins are known to play roles in neural circuit development in these regions. Psilocybin-induced modulation of semaphorin expression could therefore contribute to improved emotional regulation and stress resilience in individuals recovering from methamphetamine abuse (39). The interplay between aerobic exercise and psilocybin in modulating semaphorin gene expression offers a promising avenue for mitigating methamphetamine-induced neurotoxicity. Aerobic exercise supports neuroprotection and synaptic function through metabolic and anti-inflammatory pathways, while psilocybin enhances neuroplasticity and reduces neuroinflammation. Both interventions, by modulating semaphorin gene expression, could provide synergistic benefits in restoring neural health and function in the context of methamphetamine abuse. According to the discussed cases, the beneficial effects of exercise and psilocybin on the reduction of semaphorins 3A, 4D, and 7A were evident in rats consuming methamphetamine, and the best results were obtained when they were used simultaneously, which shows the synergistic effect of exercise and psilocybin.
Conclusion
In general, the results of the present research indicate that exercise training and psilocybin in rats using methamphetamine led to a decrease in semaphorins 3A, 4D, and 7A in the cortex of female rats. The best results were obtained in the combined group of exercise and psilocybin, which shows the synergistic effect of these two interventions. Nevertheless, the current research was associated with limitations, such as the use of female rats and the short duration of the interventions, which requires additional research for better results.
Acknowledgement
This article is taken from the thesis of the doctoral course in sports physiology, approved by Islamic Azad University, Ayatollah Amoly Branch. The authors wish to thank all the people who helped us in the progress of the work.
Funding sources
This article is extracted from a Ph.D. thesis at the Ayatollah Amoli Branch of Islamic Azad University and was personally funded by the authors.
Ethical statement
The code of ethics for the current research at Islamic Azad University, Ayatollah Amoli Branch, was reviewed and approved under the principle of ethics IR.IAU.AMOL.REC.1401.104.
Conflicts of interest
The authors have no conflicts of interest regarding the presented results.
Author contributions
FR: Original draft, Methodology. AAD: Writing, Review and Editing, Project Management. JZ: Methodology. AA: Analysis.
Research Article: Research Article |
Subject:
Sport Physiology
Received: 2024/04/28 | Accepted: 2024/09/8 | Published: 2024/12/24 | ePublished: 2024/12/24
Received: 2024/04/28 | Accepted: 2024/09/8 | Published: 2024/12/24 | ePublished: 2024/12/24
References
1. Deng B, Zhang Z, Zhou H, Zhang X, Niu S, Yan X, et al. MicroRNAs in Methamphetamine-Induced Neurotoxicity and Addiction. Front Pharmacol. 2022; 13: 875666. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007; 47: 681-98. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Baptista S, Lasgi C, Benstaali C, Milhazes N, Borges F, Fontes-Ribeiro C, et al. Methamphetamine decreases dentate gyrus stem cell self-renewal and shifts the differentiation towards neuronal fate. Stem Cell Res. 2014; 13(2): 329-41.
https://doi.org/10.1016/j.scr.2014.08.003 [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Dreyer JL. New insights into the roles of microRNAs in drug addiction and neuroplasticity. Genome Med. 2010; 2(12): 92. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Iragavarapu-Charyulu V, Wojcikiewicz E, Urdaneta A. Semaphorins in Angiogenesis and Autoimmune Diseases: Therapeutic Targets? Front Immunol. 2020; 11: 346. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Lotfi R, Yari K. The Role of Semaphorins and their Receptors in the Immune System and their Relation to Multiple Sclerosis, The Neuroscience Journal of Shefaye Khatam. 2018; 4: 75-92. [View at Publisher] [DOI] [Google Scholar]
7. Alto LT, Terman JR. Semaphorins and their Signaling Mechanisms. Methods Mol Biol. 2017; 1493: 1-25. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008; 33(4): 161-70. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Koussih L, Gounni AS. Semaphorin3E/plexinD1 Axis in Asthma: What We Know So Far! Adv Exp Med Biol. 2021; 1304: 205-213. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Loy K, Fourneau J, Meng N, Denecke C, Locatelli G, Bareyre FM. Semaphorin 7A restricts serotonergic innervation and ensures recovery after spinal cord injury. Cell Mol Life Sci. 2021; 78(6): 2911-2927. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Takegahara N, Kumanogoh A. Involvement of semaphorins and their receptors in neurological diseases, Clinical and Experimental Neuroimmunology. 2010; 1(1): 33-45.
https://doi.org/10.1111/j.1759-1961.2009.00004.x [View at Publisher] [DOI] [Google Scholar]
12. Zhao Y, Feng H, Zhang Y, Zhang JV, Wang X, Liu D, et al. Current Understandings of Core Pathways for the Activation of Mammalian Primordial Follicles. Cells. 2021; 10(6): 1491.
https://doi.org/10.3390/cells10061491 [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Shkooh HP ,Saghebjoo M, Nazemi S, Hedayati M. The Effect of One-Time and Two-Times Endurance Training with the Same Volume on Glial Cell-Derived Neurotrophic Factor and Nuclear Factor-kB in Sensory Roots of Spinal Cord in Diabetic Neuropathic Rats. Sport Physiology. 2019; 43: 75-90. [View at Publisher] [DOI] [Google Scholar]
14. Bahreini pour MA. Investigation the effect of low-intensity aerobic training for 10 weeks along with blood flow restriction on amount of protein BDNF in soleus and EDL muscles as well as the sciatic nerve in aged male rats. Journal of Sport and Exercise Physiology. 2019; 12(1): 59-75. [View at Publisher] [DOI] [Google Scholar]
15. G. Candow, Forbes SC, Chilibeck PD, Cornish SM, Antonio RB. Kreider, Effectiveness of creatine supplementation on aging muscle and bone: focus on falls prevention and inflammation. J. Clin. Med. 2019; 8(4): 488.
https://doi.org/10.3390/jcm8040488 [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Nkadimeng SM, Nabatanzi A, Steinmann CML, Eloff JN. Phytochemical, cytotoxicity, antioxidant and anti-inflammatory effects of Psilocybe natalensis magic mushroom, Plants. 2020; 9: 1127. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Bershad AK, Preller KH, Lee R, Keedy S, Wren-Jarvis J, Bremmer MP, et al. Preliminary Report on the Effects of a Low Dose of LSD on Resting-State Amygdala Functional Connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020; 5(4): 461-467. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Curtis EK. Meth mouth: a review of methamphetamine abuse and its oral manifestations. Gen Dent. 2006; 54(2): 125-9. [View at Publisher] [PMID] [Google Scholar]
19. Hibicke M, Landry AN, Kramer HM, Talman ZK, Nichols CD. Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem Neurosci. 2020; 11(6): 864-871. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Nkadimeng SM, Steinmann CML, Eloff JN. Anti-Inflammatory Effects of Four Psilocybin-Containing Magic Mushroom Water Extracts in vitro on 15-Lipoxygenase Activity and on Lipopolysaccharide-Induced Cyclooxygenase-2 and Inflammatory Cytokines in Human U937 Macrophage Cells. J Inflamm Res. 2021; 14: 3729-3738. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Gotvaldová K, Hájková K, Borovička J, Jurok R, Cihlářová P, Kuchař M. Stability of psilocybin and its four analogs in the biomass of the psychotropic mushroom Psilocybe cubensis, Drug testing and analysis. 2021; 2: 439-446. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Zhang KK, Wang H, Qu D, Chen LJ, Wang LB, Li JH, et al. Luteolin Alleviates Methamphetamine-Induced Hepatotoxicity by Suppressing the p53 Pathway-Mediated Apoptosis, Autophagy, and Inflammation in Rats. Front Pharmacol. 2021; 12: 641917. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Wang J, Liang M, Shang Q, Qian H, An R, Liu H, et al. Psilocin suppresses methamphetamine-induced hyperlocomotion and acquisition of conditioned place preference via D2R-mediated ERK signaling. CNS Neurosci Ther. 2023; 29(3): 831-841. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Marques E, Vasconcelos F, Rolo MR, Pereira FC, Silva AP, Macedo TR, et al. Influence of chronic exercise on the amphetamine-induced dopamine release and neurodegeneration in the striatum of the rat. Ann N Y Acad Sci. 2008; 1139: 222-31. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Gonçalves JR. Como fazer um projeto de pesquisa de um artigo de revisão de literature, Revista JRG de Estudos Acadêmicos. 2019; 5 01-28. [View at Publisher] [DOI] [Google Scholar]
26. Robbins B, Perry J, Long M, Carpenter RE. Analysis of D- and L- Isomers of (Meth)amphetamine in Human K2EDTA Plasma. Journal of Biomedical and Life Sciences. 2023; 3(1): 1–12. [View at Publisher] [DOI] [Google Scholar]
27. Van Battum EY, Gunput RA, Lemstra S, Groen EJ, Yu KL, Adolfs Y, et al. The intracellular redox protein MICAL-1 regulates the development of hippocampal mossy fibre connections. Nat Commun. 2014; 5: 4317. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Barcelo-Bovea V, Dominguez-Martinez I, Joaquin-Ovalle F, Amador LA, Castro-Rivera E, Medina-Álvarez K, et al. Optimization and Characterization of Protein Nanoparticles for the Targeted and Smart Delivery of Cytochrome c to Non-Small Cell Lung Carcinoma. Cancers (Basel). 2020; 12(5): 1215. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Moretti S, Procopio A, Lazzarini R, Rippo MR, Testa R, Marra M, Tamagnone L, Catalano A. Semaphorin3A signaling controls Fas (CD95)-mediated apoptosis by promoting Fas translocation into lipid rafts. Blood. 2008; 111(4): 2290-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Fazelzadeh M, Afzalpour ME, Fallah Mohammadi Z, Falah Mohammadi H. The effects of voluntary complex and regular wheel running exercises on the levels of 8-oxoguanine DNA glycosylase, semaphorin 3B, H2O2, and apoptosis in the hippocampus of diabetic rats. Brain Behav. 2021; 11(3): e01988. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Radak Z, Toldy A, Szabo Z, Siamilis S, Nyakas C, Silye G, Jakus J, Goto S. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int. 2006; 49(4): 387-92. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009; 32(5): 283-90. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Kwak HB, Thalacker-Mercer A, Anderson EJ, Lin CT, Kane DA, Lee NS, et al. Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal myotubes. Free Radic Biol Med. 2012; 52(1): 198-207. [View at Publisher] [DOI] [PMID] [Google Scholar]
34. M Alipour. The effect of intellectual capital on firm performance: an investigation of Iran insurance companies, Measuring Business Excellence. 2012; 1: 53-66. [View at Publisher] [DOI] [Google Scholar]
35. Ghadiri Hormati L, Aminaei M, Dakhili A B, Asadi shekaari M. The Effect of High-Intensity Exercise Training on Gene Expression of Semaphorin 3A in Extensor Digitorum Longus Muscles of Aged C57bl/6 Mice . J. Ilam Uni. Med. Sci. 2017; 25 (1) :92-102 [View at Publisher] [DOI] [Google Scholar]
36. Lieberz J, Shamay-Tsoory SG, Saporta N, Esser T, Kuskova E, Stoffel-Wagner B, et al. Loneliness and the Social Brain: How Perceived Social Isolation Impairs Human Interactions. Adv Sci (Weinh). 2021; 8(21): e2102076.
https://doi.org/10.1002/advs.202102076 [View at Publisher] [DOI] [PMID] [Google Scholar]
37. Gunaydin LA, Deisseroth K. Dopaminergic Dynamics Contributing to Social Behavior. Cold Spring Harb Symp Quant Biol. 2014;79:221-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
38. Wang Z, Zheng R, Wang X, Huang X, Huang J, Gu C, et al. Aerobic Exercise Improves Methamphetamine-Induced Olfactory Dysfunction Through α-Synuclein Intervention in Male Mice. Front Mol Neurosci. 2022; 15: 884790. [View at Publisher] [DOI] [PMID] [Google Scholar]
39. Xu J, Zhu Z, Jin Y, Wei C, Wang Y, Li X. Effect of aerobic exercise on brain metabolite profiles in the mouse models of methamphetamine addiction: LC-MS-based metabolomics study. BMC Psychiatry. 2023; 23(1): 852. [View at Publisher] [DOI] [PMID] [Google Scholar]
40. Coppola M, Bevione F, Mondola R. Psilocybin for Treating Psychiatric Disorders: A Psychonaut Legend or a Promising Therapeutic Perspective? J Xenobiot. 2022; 12(1): 41-52. [View at Publisher] [DOI] [PMID] [Google Scholar]
41. Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016; 30(12): 1165-1180.
https://doi.org/10.1177/0269881116675512 [View at Publisher] [DOI] [PMID] [Google Scholar]
42. M. P. Bogenschutz, A. A. Forcehimes, J. A. Pommy, C. E. Wilcox, P. C. R. Barbosa, R. J Strassman, Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study, Journal of psychopharmacology. 3 (2015) 289-299,
https://doi.org/10.1177/0269881114565144 [DOI] [PMID]
43. du Jardin KG, Liebenberg N, Cajina M, Müller HK, Elfving B, Sanchez C, Wegener G. S-Ketamine Mediates Its Acute and Sustained Antidepressant-Like Activity through a 5-HT1B Receptor Dependent Mechanism in a Genetic Rat Model of Depression. Front Pharmacol. 2018; 8: 978. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com