Volume 19, Issue 4 (Jul-Aug 2025)
mljgoums 2025, 19(4): 18-23 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
VJ K, Raman A, Ramamoorthi S, Gochhait D, Jinkala S. Comparative study of microwave tissue processing versus conventional tissue processing. mljgoums 2025; 19 (4) :18-23
URL: http://mlj.goums.ac.ir/article-1-1678-en.html
URL: http://mlj.goums.ac.ir/article-1-1678-en.html
1- Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry, India
2- Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry, India ,sree.path177@gmail.com
2- Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry, India ,
Full-Text [PDF 740 kb]
(383 Downloads)
| Abstract (HTML) (1098 Views)
Results
The quality of three different tissue processing methods was evaluated based on histomorphology using H&E staining on 89 samples.
CTP versus LMP
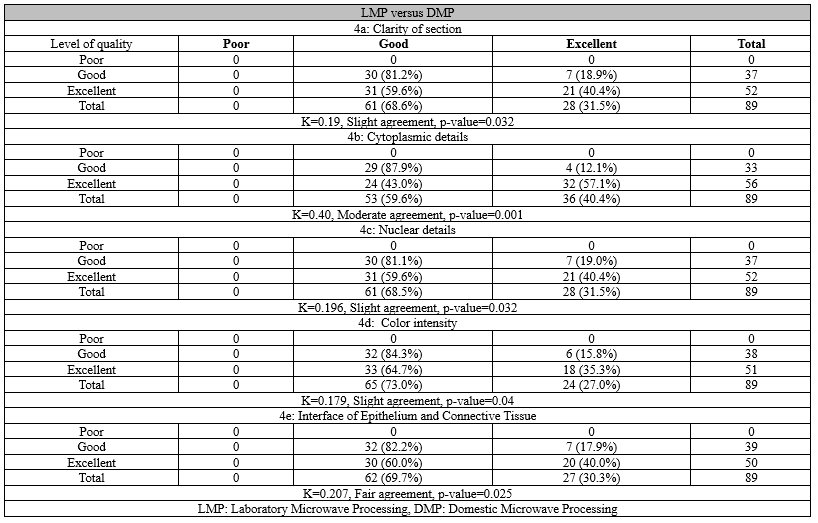
For parameters like Cytoplasmic details, nuclear details, and color intensity, LMP is superior to CTP (Figure 1). For clarity of section CTP and LMP got an equally good result. In terms of the interface of epithelium and connective tissue, CTP is better. Overall, there was a fair to moderate agreement between the CTP and LMP methods for various histomorphological features examined (Table 2).
Discussion
Most studies on MTP compare CTP with DMP. Only a few studies in the literature compare CTP with LMP. In this study, the protocol for DMP followed established methods from similar studies (1,5). A Samsung microwave oven (Model No. MS23F301T) was used, operating at a minimum power level of 100 W despite its maximum output of 800 W. Processing tissues at a low power level helps prevent damage, which aligns with previous findings (5-7). Notably, microwave processing was significantly faster (48 minutes for LMP and 2 hours for DMP) than CTP (16 hours), enabling same-day diagnosis-a result consistent with other research (1,4-7).
Our study demonstrated that the histomorphological outcomes of LMP were highly comparable to those of CTP, showing fair to moderate agreement. These findings support earlier studies (8-10). For instance, one study comparing LMP and CTP in 158 paired tissues found no substantial difference in overall section quality (11). The superior results of LMP in our study can be attributed to uniform heat distribution and vacuum-assisted processing, which enhance tissue preservation. In contrast, CTP versus DMP showed only slight to fair agreement, indicating that DMP yielded poorer histomorphological outcomes than CTP. This contrasts with some studies that reported superior or similar results for DMP (1,5,6).
Additionally, our study revealed that DMP histomorphological results were comparable to those of LMP. The enhanced performance of LMP can be explained by the agitation and vacuum features absent in DMP. To our knowledge, no prior studies have directly compared DMP and LMP, making this the first such comparison. For immunohistochemistry (IHC) staining, CTP, LMP, and DMP all exhibited excellent staining for parameters such as background clarity and antigen localization. All three methods performed equally well in terms of staining crispness. However, staining intensity was better in CTP and LMP than in DMP. Another study reported similar IHC staining quality between LMP and CTP (10), supporting our observations.
Overall, our findings indicate that LMP produces histomorphological and immunohistochemical results on par with CTP, while outperforming DMP. Microwave processing offers additional advantages, including reduced processing time, lower reagent costs, and the elimination of harmful substances such as formalin and xylene. Thus, MTP achieves three key goals: faster processing, cost efficiency, and reduced toxicity.
Strengths of the study
Conclusion
LMP yields histomorphological and immunohistochemical results comparable to CTP. DMP, however, produced slightly inferior histomorphological outcomes compared to both CTP and LMP. Nevertheless, all three techniques performed equally well in IHC staining.
Acknowledgement
We would like to acknowledge the help and support by Mrs. Kalaivizhi during the conduct of this study.
Funding sources
The study was supported by the intramural fund at our institute.
Ethical statement
The Institute’s Ethics Committee has granted waiver of consent for this study (JIP/IEC/2021/095).
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
Mrs, Karthika VJ: Execution of the study, Review of literature, Analysis and Manuscript writing. Arthy Raman: Execution of the study and Interpretation of slides. Subhashini Rammoorthi: Execution of study and Interpretation of slides. Debasis Gochhait: Interpretation of slides and Revision of manuscript. SreeRekha Jinkala: Conceptualization and Design of study, Result analysis and Manuscript writing.
Data availability statement
Available on a reasonable request.
Full-Text: (161 Views)
Introduction
Conventional Tissue Processing (CTP), which includes fixation, dehydration, clearing, and impregnation, has been the gold standard method used for centuries. It takes a lot of time to complete these steps in conventional practice. Early diagnosis and treatment, rapid tissue processing, and staining of histologic specimens are of utmost importance (1,2).
Rapid tissue processing for histopathological diagnosis has become more desirable in recent years. The use of microwaves in histopathology has become more widespread over the last decade (3). In contrast to conventional heating, microwaves create heat from within, evenly warming the tissue specimen and allowing chemicals to diffuse more rapidly. The benefits of microwave processing include reduced turnaround time and histomorphologies that are comparable to those produced by CTP (4).
Many studies have highlighted the importance of MTP and showed that tissue processed in a microwave tissue processor gives comparable morphology on histology and immunohistochemistry as conventionally processed tissue. In this study, we investigated whether Microwave Tissue Processing (MTP) (Laboratory and domestic) can provide the same quality as CTP in terms of histomorphology and immunohistochemistry staining in a shorter time.
Methods
This cross-sectional study was conducted in a histopathology laboratory at a tertiary health care centre in South India over a one-year period. The sample size was 89 for each tissue processing method using nMaster 2.0 software. A waiver of consent was obtained from the Institute’s Ethics Committee (JIP/IEC/2021/095).
The resection specimens were fixed in 10% neutral buffered formalin for 24 hours, then three tissue bits were taken from each specimen, measuring 0.5×2×2cm. One bit was processed by the CTP method, labelled as CTP. The second tissue bit was labelled as LMP, which is processed by a laboratory microwave tissue processor, and the third bit labelled as DMP was processed by using a domestic microwave oven. After tissue processing and embedding in paraffin, all three sections were cut and stained with Hematoxylin and Eosin (H and E) stain. In subgroup analysis, immunohistochemistry (IHC) was performed on 17 cases. Using two antibodies, Ki67 (Nuclear) and Pancytokeratin (Membranous and cytoplasmic), were used for IHC on relevant tissues. The Leica TP1020 open-type tissue processor was used for CTP, and its turnaround time was around 18 hours.
Laboratory Microwave Processing (LMP)
For LMP, it was standardised first according to the Thermo Scientific tissue wave microwave processing protocol. Thermo Scientific (Model No: 3486 M) Shandon Tissue Wave 2 was used for processing. Up to 74 cassettes can be processed at a time in this microwave processor. During the study, we processed only 20-30 cassettes at a time. The processing time was 48 minutes, and the schedule can be found in Table 1.
Domestic Microwave Processing (DTP)
The Samsung MS23F301T microwave oven with a capacity of 23L, was used. The microwave oven was operated at a minimum power level of 100W. The tissue was placed in plastic cassettes and placed in a 500 ml glass beaker containing reagents. The opening of the jar was covered with aluminum foil. The beaker is then placed in the centre of the rotating table in the microwave oven. The tissues were processed in a microwave in four steps: two 30-minute intervals in isopropyl alcohol, followed by two 30-minute intervals in paraffin wax. The comparison between CTP versus LMP, CTP versus DMP, and LMP versus DMP in terms of histomorphology and IHC staining was done by two pathologists independently based on the following parameters as poor (Score 0), good (Score 1), and excellent (Score 2). They were blinded to the tissue processing method to avoid bias in interpretation.
Parameters analyzed on histomorphology included clarity of section, cytoplasmic details, nuclear details, colour intensity of staining, and interface of epithelium and connective tissue (IECT). Parameters analyzed on the morphology of IHC included crispness of staining, localization of antigen, intensity of staining, and background staining.
Statistical analysis
The data were analyzed using SPSS 19.0 software, and the scores that were obtained for all three techniques were presented as a frequency distribution. The agreement between the three methods was analyzed by Kappa statistics.
Conventional Tissue Processing (CTP), which includes fixation, dehydration, clearing, and impregnation, has been the gold standard method used for centuries. It takes a lot of time to complete these steps in conventional practice. Early diagnosis and treatment, rapid tissue processing, and staining of histologic specimens are of utmost importance (1,2).
Rapid tissue processing for histopathological diagnosis has become more desirable in recent years. The use of microwaves in histopathology has become more widespread over the last decade (3). In contrast to conventional heating, microwaves create heat from within, evenly warming the tissue specimen and allowing chemicals to diffuse more rapidly. The benefits of microwave processing include reduced turnaround time and histomorphologies that are comparable to those produced by CTP (4).
Many studies have highlighted the importance of MTP and showed that tissue processed in a microwave tissue processor gives comparable morphology on histology and immunohistochemistry as conventionally processed tissue. In this study, we investigated whether Microwave Tissue Processing (MTP) (Laboratory and domestic) can provide the same quality as CTP in terms of histomorphology and immunohistochemistry staining in a shorter time.
Methods
This cross-sectional study was conducted in a histopathology laboratory at a tertiary health care centre in South India over a one-year period. The sample size was 89 for each tissue processing method using nMaster 2.0 software. A waiver of consent was obtained from the Institute’s Ethics Committee (JIP/IEC/2021/095).
The resection specimens were fixed in 10% neutral buffered formalin for 24 hours, then three tissue bits were taken from each specimen, measuring 0.5×2×2cm. One bit was processed by the CTP method, labelled as CTP. The second tissue bit was labelled as LMP, which is processed by a laboratory microwave tissue processor, and the third bit labelled as DMP was processed by using a domestic microwave oven. After tissue processing and embedding in paraffin, all three sections were cut and stained with Hematoxylin and Eosin (H and E) stain. In subgroup analysis, immunohistochemistry (IHC) was performed on 17 cases. Using two antibodies, Ki67 (Nuclear) and Pancytokeratin (Membranous and cytoplasmic), were used for IHC on relevant tissues. The Leica TP1020 open-type tissue processor was used for CTP, and its turnaround time was around 18 hours.
Laboratory Microwave Processing (LMP)
For LMP, it was standardised first according to the Thermo Scientific tissue wave microwave processing protocol. Thermo Scientific (Model No: 3486 M) Shandon Tissue Wave 2 was used for processing. Up to 74 cassettes can be processed at a time in this microwave processor. During the study, we processed only 20-30 cassettes at a time. The processing time was 48 minutes, and the schedule can be found in Table 1.
Domestic Microwave Processing (DTP)
The Samsung MS23F301T microwave oven with a capacity of 23L, was used. The microwave oven was operated at a minimum power level of 100W. The tissue was placed in plastic cassettes and placed in a 500 ml glass beaker containing reagents. The opening of the jar was covered with aluminum foil. The beaker is then placed in the centre of the rotating table in the microwave oven. The tissues were processed in a microwave in four steps: two 30-minute intervals in isopropyl alcohol, followed by two 30-minute intervals in paraffin wax. The comparison between CTP versus LMP, CTP versus DMP, and LMP versus DMP in terms of histomorphology and IHC staining was done by two pathologists independently based on the following parameters as poor (Score 0), good (Score 1), and excellent (Score 2). They were blinded to the tissue processing method to avoid bias in interpretation.
Parameters analyzed on histomorphology included clarity of section, cytoplasmic details, nuclear details, colour intensity of staining, and interface of epithelium and connective tissue (IECT). Parameters analyzed on the morphology of IHC included crispness of staining, localization of antigen, intensity of staining, and background staining.
Statistical analysis
The data were analyzed using SPSS 19.0 software, and the scores that were obtained for all three techniques were presented as a frequency distribution. The agreement between the three methods was analyzed by Kappa statistics.
|
Table 1. Processing schedule for laboratory microwave oven
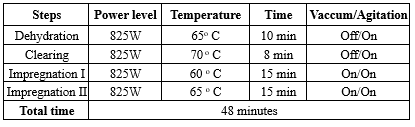 |
Results
The quality of three different tissue processing methods was evaluated based on histomorphology using H&E staining on 89 samples.
CTP versus LMP
For parameters like Cytoplasmic details, nuclear details, and color intensity, LMP is superior to CTP (Figure 1). For clarity of section CTP and LMP got an equally good result. In terms of the interface of epithelium and connective tissue, CTP is better. Overall, there was a fair to moderate agreement between the CTP and LMP methods for various histomorphological features examined (Table 2).
 Figure 1. Comparison of histomorphology of a slide of squamous cell carcinoma - Conventional tissue processing (a) and laboratory microwave processing (b) show equally good morphology with a fair to moderate agreement (H and E 100x) |
CTP versus DMP
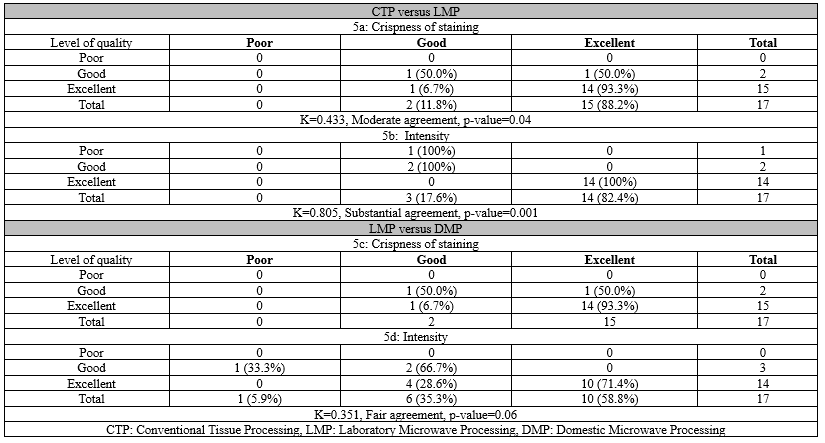
For parameters like cytoplasmic details and interface of epithelium, the results of DMP were slightly poor, and for other parameters like the clarity of section, color intensity, and nuclear details, the results of DMP were poor when compared to CTP (Figure 2). Overall, there was a slight to fair agreement between the CTP and DMP methods for various histomorphological parameters assessed, with CTP having a higher frequency of excellent scores (Table 3).
LMP versus DMP
In our study, the clarity of section, cytoplasmic details, nuclear details, colour intensity, and interface of epithelium and connective tissue were found to be better in LMP when compared to DMP (Figure 3). Overall, there was a slight to fair agreement between the LMP and DMP methods for various histomorphological parameters (Table 4).
For parameters like cytoplasmic details and interface of epithelium, the results of DMP were slightly poor, and for other parameters like the clarity of section, color intensity, and nuclear details, the results of DMP were poor when compared to CTP (Figure 2). Overall, there was a slight to fair agreement between the CTP and DMP methods for various histomorphological parameters assessed, with CTP having a higher frequency of excellent scores (Table 3).
LMP versus DMP
In our study, the clarity of section, cytoplasmic details, nuclear details, colour intensity, and interface of epithelium and connective tissue were found to be better in LMP when compared to DMP (Figure 3). Overall, there was a slight to fair agreement between the LMP and DMP methods for various histomorphological parameters (Table 4).
|
Table 2. Agreement between CTP versus LMP (n=89)
 |
 Figure 2. Comparison of histomorphology of a slide of normal liver parenchyma - Conventional tissue processing (a) and Domestic microwave processing (b) show good morphology in conventional tissue processing. The agreement was slight to fair (H and E 100x) Table 3. Agreement between CTP and DMP (n=89)  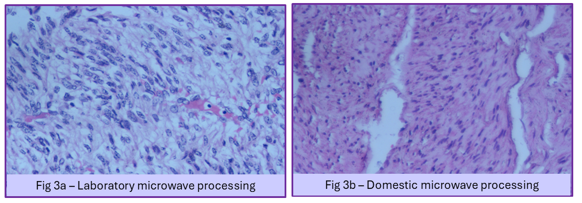 Figure 3. Comparison of histomorphology of a slide of leiomyoma uterus - Laboratory microwave processing (a) and Domestic microwave processing (b) shows good morphology on laboratory microwave processing. The agreement was slight to fair (H and E 100x) |
IHC staining: CTP versus LMP and LMP versus DMP
IHC was done on 17 cases in each group (17x3=51 specimens) using two antibodies. The comparison of IHC staining of CTP and LMP methods, LMP and DMP methods (Table 5) in terms of four different parameters in IHC-stained slides was done (Figure 4). A detailed comparison of DMP and LMP with existing literature is provided in Table 6 and 7.
IHC was done on 17 cases in each group (17x3=51 specimens) using two antibodies. The comparison of IHC staining of CTP and LMP methods, LMP and DMP methods (Table 5) in terms of four different parameters in IHC-stained slides was done (Figure 4). A detailed comparison of DMP and LMP with existing literature is provided in Table 6 and 7.
- Localisation of antigen and background staining had consensus in all 17 cases, so kappa statistics were not performed.
- The parameters, localization of antigen, and background staining showed a 100% agreement with a p-value of <0.001, which means there is no background staining and localization of antigen is very good for IHC-stained slides by CTP and LMP methods, LMP and DMP methods.
 Figure 4. Comparison of IHC of a slide of adenocarcinoma with Pancytokeratin - Conventional tissue processing (a), laboratory microwave processing (b), and Domestic microwave processing (c) shows equally good intensity and localization of antigen (DAB 100x) Table 6. Comparison of domestic microwave tissue processing schedule of similar studies with our study  Table 7. Comparison of laboratory microwave tissue processing schedule results of similar studies with our study  |
Discussion
Most studies on MTP compare CTP with DMP. Only a few studies in the literature compare CTP with LMP. In this study, the protocol for DMP followed established methods from similar studies (1,5). A Samsung microwave oven (Model No. MS23F301T) was used, operating at a minimum power level of 100 W despite its maximum output of 800 W. Processing tissues at a low power level helps prevent damage, which aligns with previous findings (5-7). Notably, microwave processing was significantly faster (48 minutes for LMP and 2 hours for DMP) than CTP (16 hours), enabling same-day diagnosis-a result consistent with other research (1,4-7).
Our study demonstrated that the histomorphological outcomes of LMP were highly comparable to those of CTP, showing fair to moderate agreement. These findings support earlier studies (8-10). For instance, one study comparing LMP and CTP in 158 paired tissues found no substantial difference in overall section quality (11). The superior results of LMP in our study can be attributed to uniform heat distribution and vacuum-assisted processing, which enhance tissue preservation. In contrast, CTP versus DMP showed only slight to fair agreement, indicating that DMP yielded poorer histomorphological outcomes than CTP. This contrasts with some studies that reported superior or similar results for DMP (1,5,6).
Additionally, our study revealed that DMP histomorphological results were comparable to those of LMP. The enhanced performance of LMP can be explained by the agitation and vacuum features absent in DMP. To our knowledge, no prior studies have directly compared DMP and LMP, making this the first such comparison. For immunohistochemistry (IHC) staining, CTP, LMP, and DMP all exhibited excellent staining for parameters such as background clarity and antigen localization. All three methods performed equally well in terms of staining crispness. However, staining intensity was better in CTP and LMP than in DMP. Another study reported similar IHC staining quality between LMP and CTP (10), supporting our observations.
Overall, our findings indicate that LMP produces histomorphological and immunohistochemical results on par with CTP, while outperforming DMP. Microwave processing offers additional advantages, including reduced processing time, lower reagent costs, and the elimination of harmful substances such as formalin and xylene. Thus, MTP achieves three key goals: faster processing, cost efficiency, and reduced toxicity.
Strengths of the study
- Both LMP and DMP methods of tissue processing drastically reduced the turnaround time.
- The CTP method and LMP method maintained comparable quality in the following aspects: clarity of section, cytoplasmic details, nuclear details, staining intensity, and the interface of epithelium and connective tissue.
- Isopropyl alcohol was used for dehydration and clearing steps in MTP, thereby reducing environmental pollution/exposure to laboratory personnel.
Limitations of the study
- There was no temperature control panel in the domestic microwave to maintain temperature during tissue processing.
- During MTP, there was evaporation of reagents and hence they cannot be reused. This results in an excess reagent requirement as compared to CTP.
Conclusion
LMP yields histomorphological and immunohistochemical results comparable to CTP. DMP, however, produced slightly inferior histomorphological outcomes compared to both CTP and LMP. Nevertheless, all three techniques performed equally well in IHC staining.
Acknowledgement
We would like to acknowledge the help and support by Mrs. Kalaivizhi during the conduct of this study.
Funding sources
The study was supported by the intramural fund at our institute.
Ethical statement
The Institute’s Ethics Committee has granted waiver of consent for this study (JIP/IEC/2021/095).
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
Mrs, Karthika VJ: Execution of the study, Review of literature, Analysis and Manuscript writing. Arthy Raman: Execution of the study and Interpretation of slides. Subhashini Rammoorthi: Execution of study and Interpretation of slides. Debasis Gochhait: Interpretation of slides and Revision of manuscript. SreeRekha Jinkala: Conceptualization and Design of study, Result analysis and Manuscript writing.
Data availability statement
Available on a reasonable request.
Research Article: Original Paper |
Subject:
Laboratory Sciences
Received: 2023/06/6 | Accepted: 2024/05/20 | Published: 2025/09/17 | ePublished: 2025/09/17
Received: 2023/06/6 | Accepted: 2024/05/20 | Published: 2025/09/17 | ePublished: 2025/09/17
References
1. Amrutha N, Patil S, Rao RS. Microwaves: a revolution in histoprocessing. J Contemp Dent Pract. 2014:15(2);149-52. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Tosuner Z, Karaer O, İnce Ü. Lean Six Sigma Applications in a Multicenter Pathology Laboratory: An Industrial Pathology Model. Research. 2023:4(55). [View at Publisher] [DOI] [Google Scholar]
3. Mathai AM, Naik R, Pai MR, Rai S, Baliga P. Microwave histoprocessing versus conventional histoprocessing. Indian J Pathol Microbiol. 2008; 51(1):12-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Chandy D, Swetha D'Souza P, Jeyalyn David S. Tissue Processing using Microwave Oven: A Boon for Histology Slide Preparation. International Journal of Anatomy and Research. 2024;12(2):8903-09. [View at Publisher] [DOI]
5. Patil S, Rao RS, Nagaraja A, Kumar SD. Comparison of conventional and microwave histo-processing of various oral soft tissue specimens. Journal of Dental Research and Review. 2014;1(1):3-6. [View at Publisher] [DOI] [Google Scholar]
6. Rao M, Pai SM, Khanagar SB, Siddeeqh S, Devang DD, Naik S. Microwave-assisted tissue processing, fixation and staining in tissues of different thicknesses: A comparative study. J Oral Maxillofac Pathol. 2020;24(1):186. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Bhuvanamha devi R, Subhasree AR, Parameaswari PJ, Parijatham BO . Domestic microwave versus conventional tissue processing: A quantitative and qualitative analysis. J Clin Diagn Res. 2013;7(5):835-39. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Shrestha G, Karki S, Pradhan A. Changing perspective on tissue processing-comparison of microwave histoprocessing method with the conventional method. Journal of Pathology of Nepal. 2015;5(10):841-6. [View at Publisher] [DOI] [Google Scholar]
9. Rohr LR, Layfield LJ, Wallin D, Hardy D. A comparison of routine and rapid microwave tissue processing in a surgical pathology laboratory: quality of histologic sections and advantages of microwave processing. Am J Clin Pathol. 2001;115(5):703-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Emerson LL, Tripp SR, Baird BC, Layfield LJ, Rohr LR. A comparison of immunohistochemical stain quality in conventional and rapid microwave processed tissues. Am J Clin Pathol. 2006;125(2):176-83. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Rohr LR, Layfield LJ, Wallin D, Hardy D. A comparison of routine and rapid microwave tissue processing in a surgical pathology laboratory: quality of histologic sections and advantages of microwave processing. Am J Clin Pathol. 2001;115(5):703-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








 goums.ac.ir
goums.ac.ir yahoo.com
yahoo.com